Plaquenil (hydroxychloroquine sulfate, Sanofi-Aventis) and the less-used chloroquine are antimalarial drugs with anti-inflammatory properties that are used for the management of a spectrum of inflammatory conditions. Plaquenil is less toxic than chloroquine; however, long-term use of either drug can result in macular toxicity, leading to devastating irreversible vision loss. The mechanism of this toxicity is not clearly understood, though it is believed that the drug molecule binds to melanin in the retinal pigment epithelium (RPE).1 This leads to disruption and damage to the photoreceptors and outer nuclear and plexiform layer, sparing the foveal center and resulting in the “bull’s eye” appearance in the late stage of the disease. Binding of the drug to melanin in the RPE contributes to, or prolongs, its toxic effects.2
Plaquenil toxicity is typically asymptomatic in early stages, but over time can lead to severe vision loss and retinal damage. The risk of retinal toxicity was initially believed to be less than 1% after long-term (or a cumulative dosage of 1,000mg).3 The toxicity would double by 10 years, resulting in a 20% prevalence after 20 years.4 However, research now shows toxicity continues even after cessation of the drug.5 Thus preventing and detecting early effects of retinal toxicity prior to irreversible complications is key.6
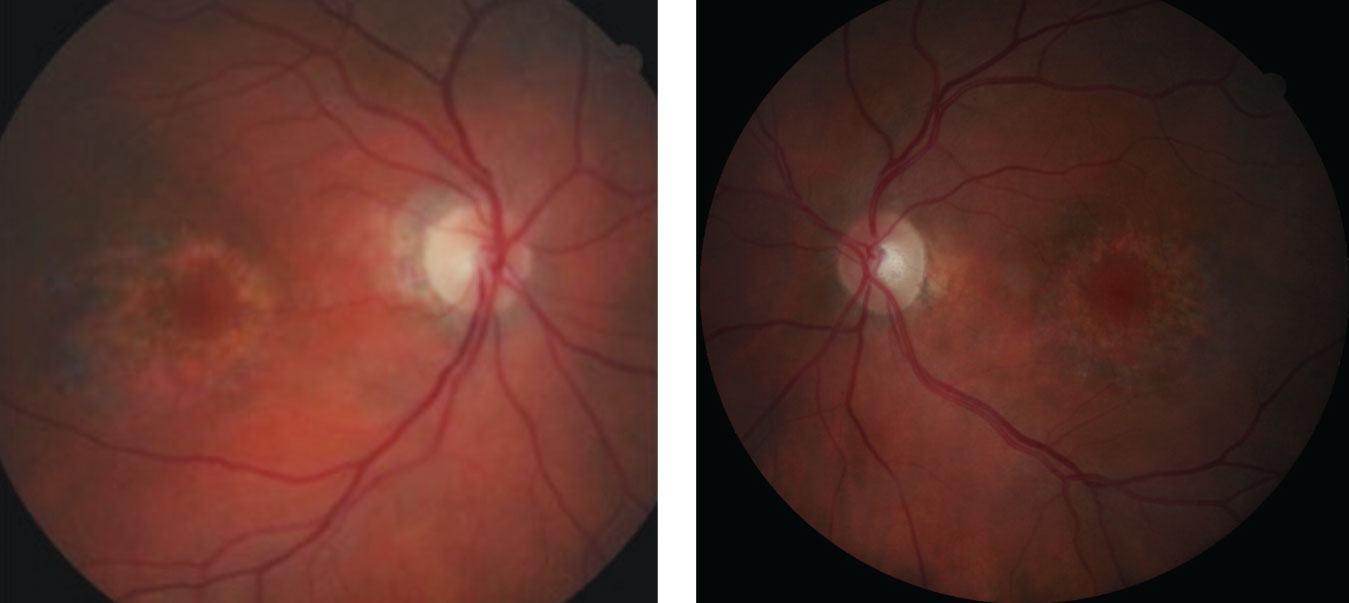 |
| Fig. 1a and 1b. These fundus photos, of the right and left eyes respectively, reveal parafoveal ring-shaped “bull’s eye” RPE defects. |
Follow the Guides
The latest screening guidelines were published in 2016 by the American Academy of Ophthalmology (Table 1). The most important risk factors are dosage and duration of use. Dosage greater than 5.0mg/kg over five years dramatically increases the risk of retinal toxicity, and high doses can be exceedingly dangerous. As opposed to the 2011 guidelines, “real weight” is now considered a better indicator than “ideal weight” for calculating dosage for most patients. For example, for the maximum safe dose of 5.0mg/kg, the threshold dose would be 400mg/day for a patient weighing 175 pounds. Other major risk factors include concomitant renal disease, concomitant retinal disease and the use of tamoxifen (Table 2).7,8
Currently, one of the primary functional screening tests recommended for the evaluation of Plaquenil retinal toxicity is 10-2 white stimulus automated visual fields; however, research shows Asian patients benefited from 24-2 or 30-2 visual fields, given that toxicity often manifests changes beyond the macula in these patients.9 Because spectral-domain optical coherence tomography (SD-OCT) is readily accessible and able to detect early structural damage prior to clinical funduscopic findings, it is now one of the primary objective screening tests. However, variable SD-OCT findings related to Plaquenil retinal toxicity can make the screening data challenging to interpret; thus, it is important that clinicians become familiar with the spectrum of SD-OCT findings.
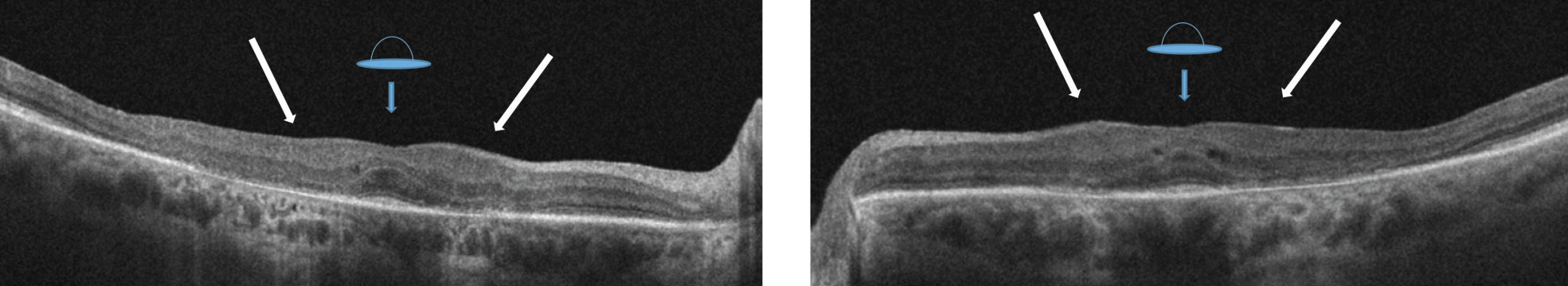 |
| Figs. 2a. and 2b. OCT of the right eye reveals a “flying saucer sign” of the macula with associated loss of the parafoveal IS/OS junction. Displacement of the inner retinal structures with loss of foveal contour unveils the “sinkhole sign” (see white arrows). |
Although multifocal electroretinogram (mfERG) and fundus autofluorescence (FAF) are not currently primary tests used in the evaluation of Plaquenil retinal toxicity, they may be beneficial when the diagnosis or findings are enigmatic or an adjunct test is warranted.
A review of the various OCT and visual field findings associated with retinal toxicity, as well as case presentations and illustrations, can help you be prepared when a patient on Plaquenil presents for an eye exam.
 |
Case One
An 87-year-old Caucasian female presented complaining of blurred vision while reading with her current prescription. Her medical history revealed age-related macular degeneration that was diagnosed approximately 30 years ago (for which she uses AREDS2 supplements) and cataract surgery in both eyes with posterior chamber intraocular lens (IOL) implementation 10 years prior. Her medical history was remarkable for hypertension, hypocholesteremia and rheumatoid arthritis. She was currently taking an unknown beta-blocker and statin QD.
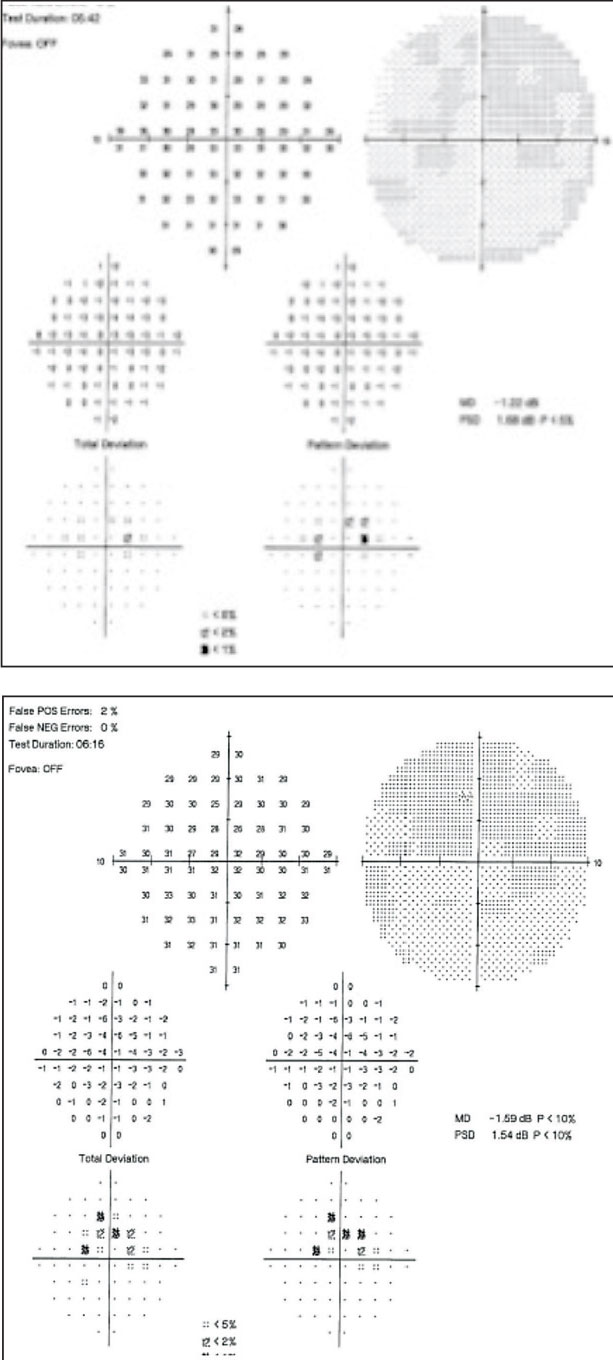
Her best-corrected acuities were 20/40+ OD and 20/30- OS. All preliminary testing, including pupils, extraocular motilities and confrontation fields, were unremarkable. Slit lamp exam only revealed posterior chamber IOLs OU. Intraocular pressures were 14mm Hg OU. A dilated fundus exam revealed unremarkable optic discs and normal physiological cupping. Parafoveal ring-shaped “bull’s eye” RPE defects in the macula of both eyes were also noted (Figures 1a and 1b). The vessels and peripheral exam did not reveal any abnormal findings in either eye. SD-OCT revealed “flying saucer” sign of the macula area with associated loss of the parafoveal IS/OS junction in both eyes, but intact subfoveally (Figures 2a and 2b). No associated drusen was noted.
The findings were suspicious for retinal toxicity, and the patient did reveal a history of Plaquenil use starting at age 30 and lasting for 15 years. She also recalled being asked to discontinue the medication due to an unspecified “retinal problem.” She was asked to return to clinic for 10-2 visual field testing and diagnosis and management were explained.
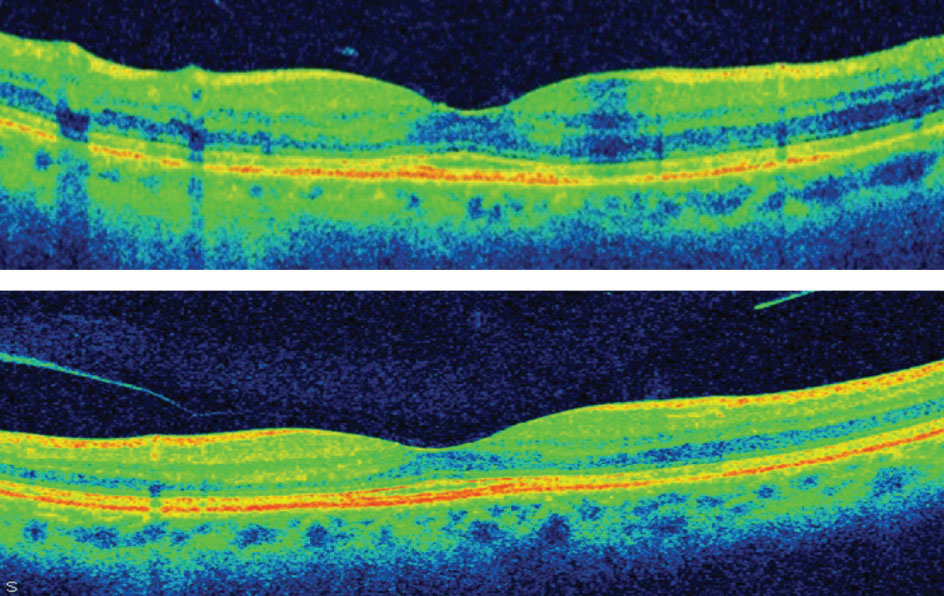 |
| Figs. 3a. and 3b. High-resolution OCT demonstrating localized parafoveal thinning in a patient with early Plaquenil toxicity. |
Case Two
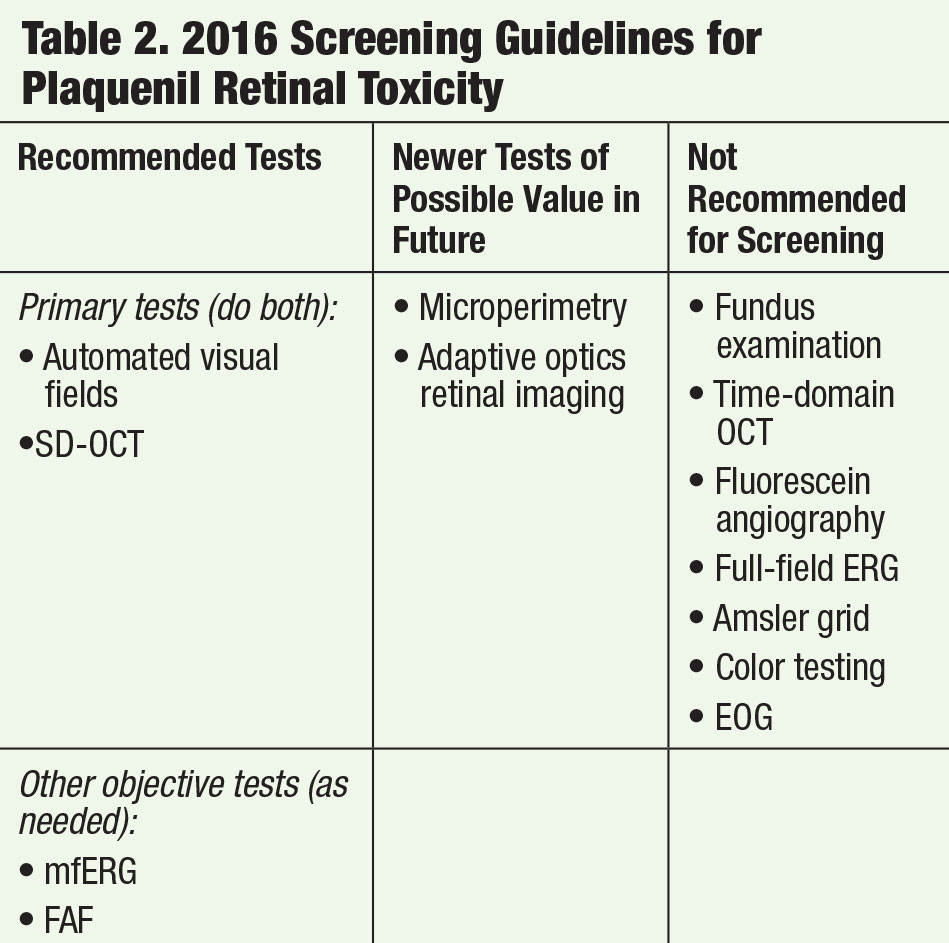
A 26-year-old black female presented in office for her annual comprehensive eye exam. Her medical history was significant for Plaquenil therapy spanning 12 years with a daily dose of 200mg twice daily. She presented with a best-corrected visual acuity of 20/20 OD and 20/20 OS. All preliminary testing revealed normal findings. Slit lamp examination was unremarkable; however, her ancillary testing revealed macular changes compatible with early toxicity. Her SD-OCT reflected localized parafoveal thinning (Figures 3a and 3b). The Humphrey visual field 10-2 (white stimulus) was reliable, showing bilateral, although asymmetric, patchy parafoveal visual field defects (Figures 4a and 4b).
She was educated on the ocular effects of Plaquenil and the continuous monitoring and management. The corresponding prescribing physician was also advised on the findings.
 |
Screening Protocol
Proper protocol implementation requires knowing when to evaluate patients on Plaquenil and what ancillary tests to perform. To start, have patients complete a questionnaire that answers the following:
- Are you currently taking Plaquenil? If yes, when did you start?
- What is your current dosage?
- What is your current weight?
- Have you previously had baseline ophthalmic testing?
- Are you currently under the care of another optometrists or ophthalmologist?
- How often do you see your rheumatologist?
- Are you being treated for kidney disease?
- Are you also taking tamoxifen?
 |
| Figs. 4a. and 4b. Visual fields of the right and left eyes reveal paracentral scotoma consistent with Plaquenil toxicity. |
A sample questionnaire is also available for download later on in this article. To properly screen patients for Plaquenil toxicity, you should have SD-OCT and automated visual fields; it is also beneficial to include FAF and mfERG. Although the latest guidelines suggest that Amsler grid, color vision and fundus photography are not considered necessary for screening patients on Plaquenil, they can still provide useful information.
What tests do you need to perform during their baseline screening? During their baseline screening, you should perform Humphrey visual field testing 10-2 (white stimulus) for non-Asian patients to detect paracentral and central scotomas.9 For Asian patients, perform a 24-2 or 30-2 visual field to detect extramacular defects with a retest using 10-2 if any defects are detected.9 SD-OCT allows early detection of damage to the IS/OS junction before you observe retinal findings. Because early toxicity changes can be revealed on SD-OCT, it is prudent to perform a baseline screening of patients taking Plaquenil. Multifocal ERG has the ability to detect early macular dysfunction, so it should be included as part of baseline screening. A maximum daily dose of Plaquenil of 5.0mg/kg real weight is recommended. Communication with the prescribing physician is also key to proper treatment and management of your patient.
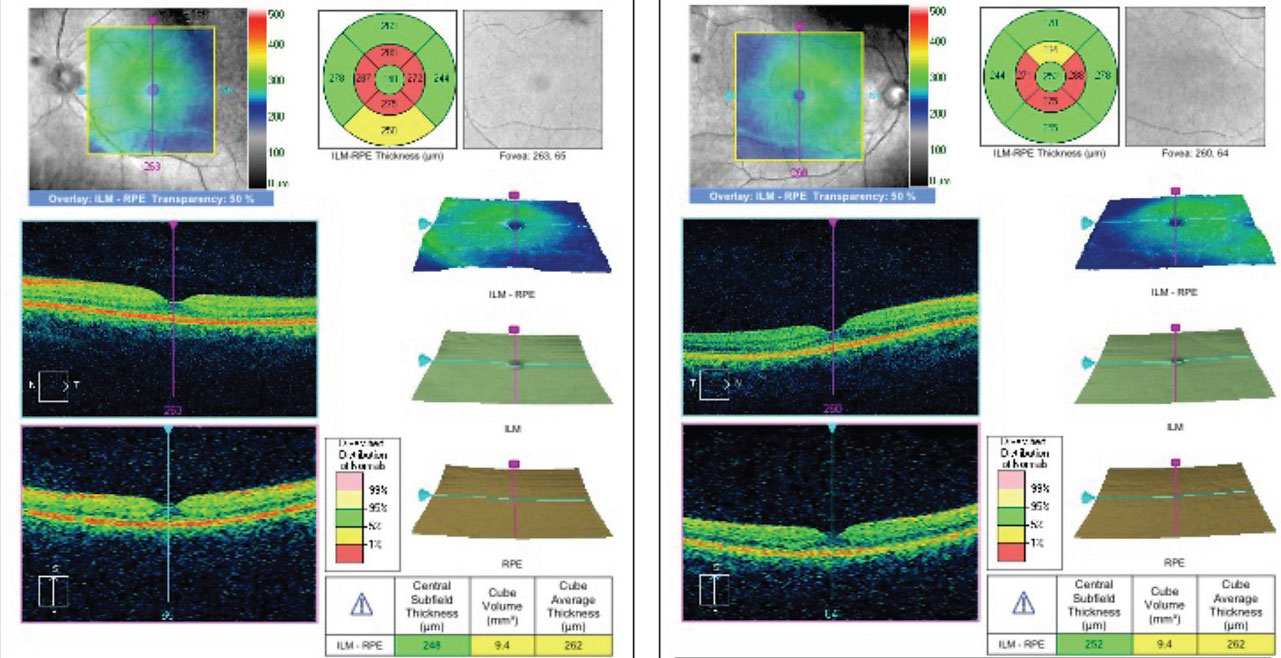 |
| Fig. 5. Early SD-OCT finding showing disruption of the parafoveal photoreceptor inner segment/outer segment junction (left). Fig. 6. Localized parafoveal thinning noted in the topographical map often correlates with IS/OS defects early in the disease course (right). |
Signs
Eyes affected by Plaquenil toxicity can be diagnosed based on one or more of several possible telltale presentations ranging from structural changes visible on imaging equipment to functional changes detectable via testing. These include:
OCT. The classic retinal toxicity has been described as “bull’s-eye maculopathy,” with the appearance of a ring of parafoveal RPE depigmentation that spares the fovea (see case presented). The presence of bull’s-eye maculopathy indicates the disease has been progressing for years, resulting in foveal thinning and likely vision loss.8 SD-OCT is a highly sensitive and reproducible imaging modality used in the detection of Plaquenil retinal toxicity. The preferential loss of photoreceptor IS/OS junction makes SD-OCT an ideal tool to identify early changes associated with Plaquenil retinal toxicity. Due to the capabilities of SD-OCT to enhance the structural assessment of the retina, it has allowed for the detection of early damage prior to funduscopic clinical findings. Yet, there are variable SD-OCT findings associated with Plaquenil retinal toxicity.
 |
| This questionnaire is designed to help identify patients with Plaquenil toxicity. Feel free to photocopy it and use it in your own practice. Click image to download. |
The earliest finding is disruption of the parafoveal photoreceptor inner segment/outer segment (IS/OS) junction, also known as ellipsoid zone or photoreceptor integrity line (Figure 5).10 This disruption is considered one of the strongest indications of early retinal toxicity.10 Early loss of the IS/OS parafoveal junction may be denoted as the attenuation of the homogenous IS/OS junction reflectivity line. This loss may also correlate with a localized parafoveal thinning in the topographical map (Figure 6). In addition, this may correlate to the paracentral scotomas often seen in patients with early toxicity. (Figure 4a and 4b)
The more moderate stage of toxicity may be followed by diffuse parafoveal outer nuclear layer (ONL) with inner plexiform layer (IPL) changes (Figure 7).10-12 Inner retina damage is often a consequence of outer retinal damage.
Disruption and loss of the external limiting membrane (ELM) may also be an early OCT finding (Figure 6).13 The ELM is theorized to play an important role in maintaining homeostasis of the photoreceptors and outer nuclear layer.14 Patients with an intact ELM at diagnosis of toxicity had a better prognosis and were unlikely to progress, whereas those with ELM disruption were more likely to show progressive changes on SD-OCT.
Increased thickness of the RPE-Bruch’s membrane complex in Plaquenil patients has also been reported as an early sign. As the drug binds to melanin in the RPE, it causes degenerative changes, which leads to alteration in RPE metabolism that contributes to the changes in the RPE-Bruch’s complex.15 Investigators believe that thickening of the outer band results from thickening of Bruch’s membrane.
 |
|
Fig. 7. High-definition OCT reveals moderate plaquenil-related damage with diffuse parafoveal ONL and IPL changes. |
The “Sinkhole” sign (Figure 2a and 2b) shows displacement of the inner retinal structures toward the retinal RPE with variable loss of the foveal contour.15 A gap between the ONL (due to ONL thinning) associated with an intact external limiting membrane contributes to a sinkhole appearance and preservation of subfoveal outer layer. The subfoveal IS/OS junction is not affected in the late stage of the disease and associated with severe loss.16
The “Flying saucer” sign. (Figure 2a and 2b) this classic representation of Plaquenil toxicity is a preservation of the outer retinal layers subfoveally with perifoveal loss of the IS/OS junction on both sides of the fovea. Perifoveal IS/OS junction loss is associated with perifoveal outer retinal thinning, resulting in an ovoid appearance in the central retina, creating the flying saucer sign. The description of this sign was helpful in identifying retinal toxicity as presented in our cases.
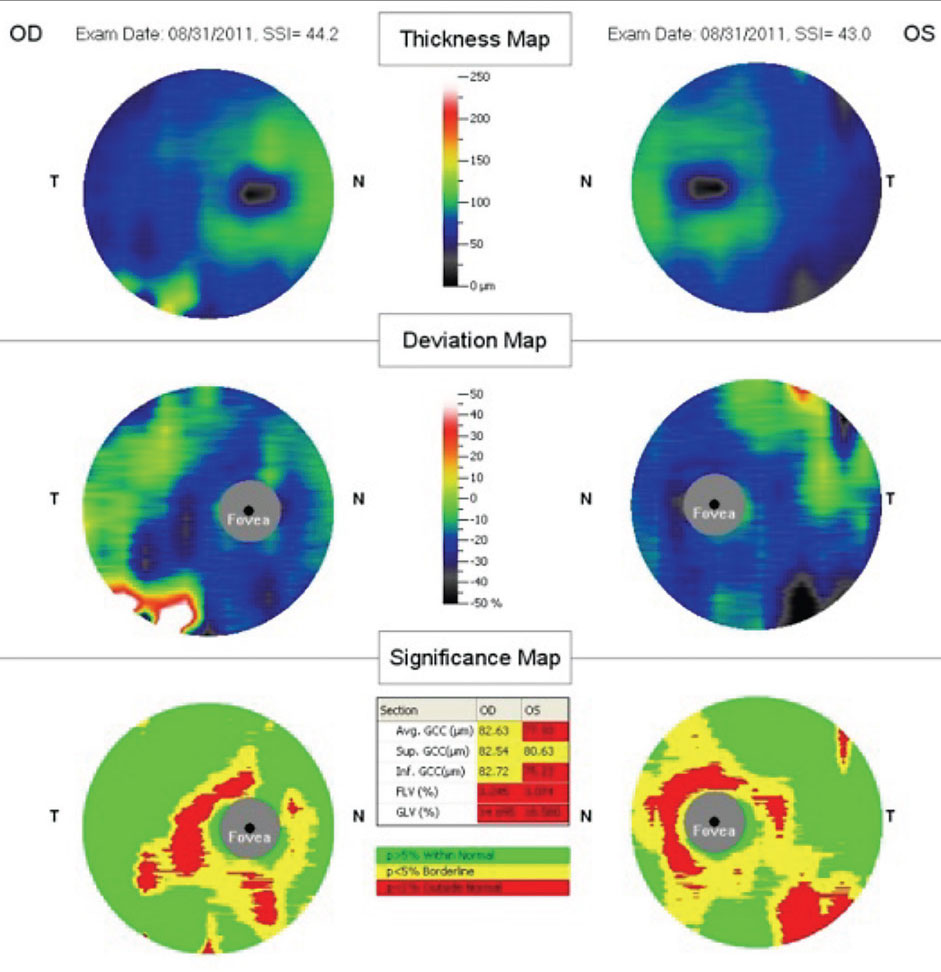
Ganglion cell complex (GCC) defect and peripapillary NFL defects (Figures 8 and 9). A 2008 report described anatomical changes associated with GCC loss within the parafoveal region, in particular within the inferior temporal region. Hence, the perifoveal thinning noted on GCC may be attributed to selective thinning of the ganglion cell layer and inner plexiform layer.17
 |
|
Fig. 8. High-definition OCT revealing disruption and loss of the external limiting membrane as early OCT findings. |
Visual fields. In early cases of Plaquenil toxicity, an early indicator of damage is the appearance of a paracentral scotoma seen on automated visual field testing in the absence of fundus changes.18 Each of the testing strategies can be used to detect early toxicity changes, but the presentation of the visual field effects will vary. Due to the central area being depressed in the 24-2 and 30-2 testing strategies, it is difficult to discern the central 2-degree field of sparing seen in the 10-2 tests. You can conceivably misinterpret the visual field if the 24-2 or 30-2 testing strategies are used, because the scotoma may appear as a small central defect as opposed to a paracentral ring scotoma.
When using the larger testing strategies, a strong indication of Plaquenil toxicity is the presentation of a scotoma in the central 4 degrees of fixation.
Researchers suggest that one central point in both eyes indicates toxicity and one should have a low threshold with regards to any defects.19 The misinterpreted visual field defect using the larger testing strategies could potentially delay the diagnosis of Plaquenil toxicity resulting in irreversible vision loss. Due to the possibility of missing the scotoma while evaluating the greyscale, observation of the total deviation and pattern deviation could help elucidate if there is early retinal toxicity.
Similar to the 24-2 and 30-2 visual fields, where the central 4 degrees are affected by Plaquenil toxicity, the area of risk on a 10-2 is two degrees to six degrees from center.19 Due to the inevitability of missed subtle defects, deviation plots should be reviewed. In the literature, it has been suggested that the superior visual field is affected first.20 Those findings were corroborated in 2011, when researchers noted the superior visual field was affected more in seven out of 15 patients.19 Evaluation of the greyscale will reveal a paracentral ring scotoma, but a subtle abnormality may be easier to detect on the pattern deviation. The choice of HVF 10-2 with the red stimulus or without the red stimulus is controversial. The 10-2 HVF with red stimulus was touted as the testing strategy of choice due to its 91% sensitivity for detection of Plaquenil toxicity; however, there is only a 57% specificity.18 On the converse, the 10-2 white stimulus testing strategy has a lower sensitivity of 78%, but it has a better specificity of 84%.
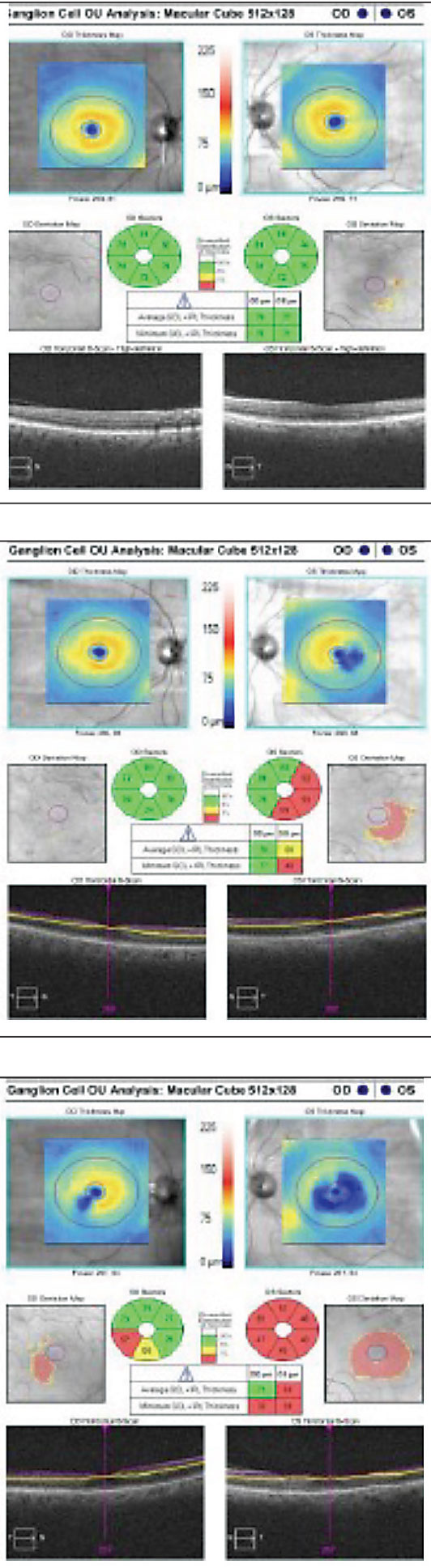 |
| Fig. 9 Cirrus SD-OCT demonstrating progressive GCC damage within the parafoveal and temporal region. |
Eye care specialists provide a valuable service when screening for Plaquenil retinal toxicity and advising the treating physician or rheumatologist with regards to the patient’s risk, safe dosing and appropriate screening procedures. With the updates of the new guidelines regarding screening for Plaquenil-related retinal toxicity, we must be more vigilant in the aggressive and thorough imaging and interpretation of such diagnostic tests. Hence, it is imperative that we become familiar with recognizing the spectrum of HVF and SD-OCT findings associated with Plaquenil retinal toxicity. Of note, SD-OCT, in combination with Humphrey visual field testing, is critical for the early detection of Plaquenil retinal toxicity.
Dr. Demeritt is an assistant professor at Nova Southeastern University College of Optometry.
Dr. Reynolds is an associate professor of optometry at Nova Southeastern University College of Optometry.
Dr. Shechtman is a professor of optometry at Nova Southeastern University College of Optometry.
Dr. Davidson is a locum optometrist and 2016 graduate of Nova Southeastern University.
1. Sundelin SP, Terman A. Different elements of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;100:481-9. 2. Gamanu (Panca) A, Popa-Cherecheanu A, Marinescu B, et al. Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. Review. J Med Life. 2014;7(3):322-6. 3. Bernstein H. Ocular safety of hydroxychloroquine sulfate (Plaquenil). South Med J. 1992;85(3):274-9. 4. Marmor M, Kellner U, Lai T, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmol. 2011;118(2):415–22. 5. Melles R, Marmor M. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453-60. 6. Michaelides M, Niam H, Stover P, et al. Retinal toxicity associated with hydroxychloroquine and chloroquine risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol. 2011;129(1):30-9. 7. Marmor M. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. 2012;130(4):461-9. 8. Marmor M, Kellner U and Lai T. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmol. 2016;123(6):1386-94. 9. Melles R, Michael F, Marmor M. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmol. 2015;122(1):110-6. 10. Lally D, Heier J, Baumal C, et al. Expanded spectral domain-OCT findings in the early detection of hydroxychloroquine retinopathy and changes following drug cessation. Int J Retina Vitreous. 2016;2:18. 11. Korah S, Kuriakose T. Optical coherence tomography in a patient with chloroquine-induced maculopathy. Indian J Ophthalmol. 2008;56(6):511-3. 12. Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009;93(11):1444-7. 13. Uslu H, Gurler B, Yildirim A, et al. Effect of hydroxychloroquine on the retinal layers: A quantitative evaluation with spectral-domain optical coherence tomography. J Ophthalmol. 2016:2016. 14. Abramoff M, Garvin M, Sonka M. Retinal imaging and image analysis. IEEE Trans Med Imaging. 2010;3(7):169-208. 15. Chen E, Brown D, Benz M, Fish R. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign) Clin Ophthalmol. 2010;4:1151–8. 16. Mititelu M, Wong B, Brenner M, Bryar P. Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol. 2013;131(9):1187-97. 17. Rodriguez-Padilla J, Hedges T, Monson B, et al. High-speed ultra-high resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007;125(6):775-80. 18. Easterbrook E. Ocular effects and safety of antimalarial agents. Am J Med. 1988;85(4.S1):23-9. 19. Anderson C, Blaha GR, Marx JL. Humphrey visual field findings in hydroxychloroquine toxicity. Eye (Lond). 2011;25(12):1535-45. 20. Hart W, Burde R, Johnston G, Drews R. Static perimertry in chloroquine retinopathy: perifoveal patterns of visual field depression. Arch Ophthalmol. 1984;102:377-80. |

