 |
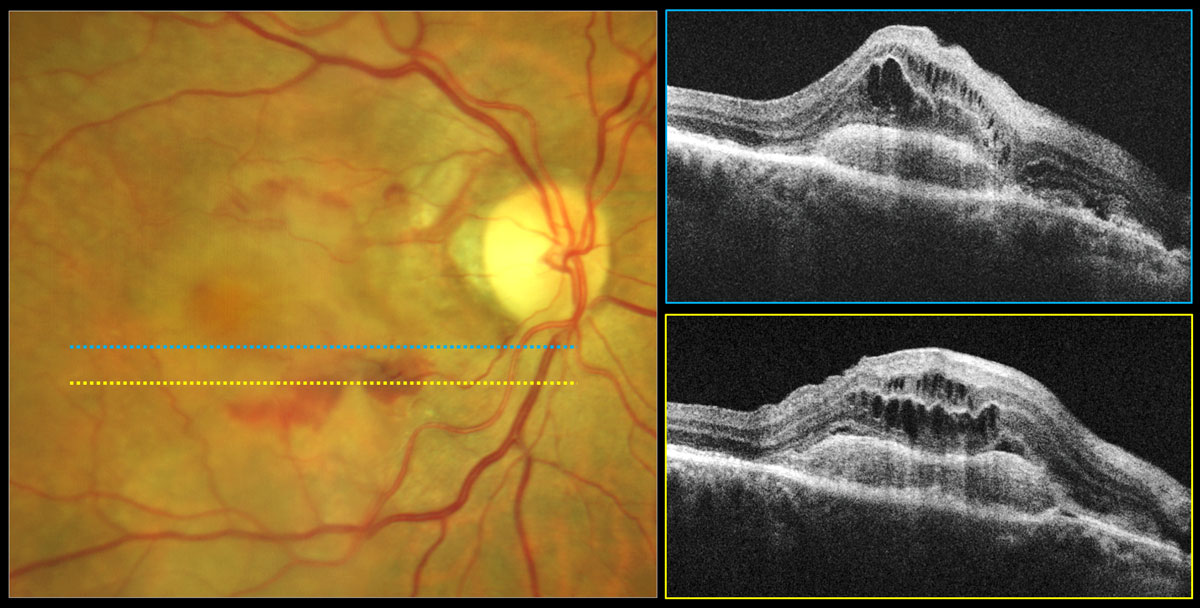
The aflibercept 8mg group displayed greater letter gain and twice as many patients experiencing no fluid at week 44 compared with the 2mg group. Photo: Carolyn Majcher, OD. Click image to enlarge. |
Estimated to affect as many as 228 million individuals globally by 2040, age-related macular degeneration (AMD) requires ongoing medical attention to stave off its worst possible outcomes. Anti-VEGF therapy has allowed millions to maintain and even regain vision but is fraught with difficulties and trade-offs, leading the pharmaceutical industry to continually seek out better options. In one new study published in JAMA Ophthalmology, this issue was addressed by the Phase II CANDELA randomized clinical trial of a high-dose form of the popular anti-VEGF drug aflibercept. Researchers posited that an 8mg dose may offer greater benefits than the present 2mg dose used currently in patients with neovascular AMD (nAMD).
CANDELA was a single-masked, open-label study conducted over 44 weeks. Treatment-naïve patients—totaling 106 eyes—with active subfoveal choroidal neovascularization secondary to nAMD and who had best-corrected visual acuity of 20/32 to 20/320 were included. Patients were then randomized 1:1 to receive three monthly doses of 8mg or 2mg of aflibercept, followed with doses at 20 and 32 weeks.
Percentage of eyes without fluid in the central subfield at week 16 was 50.9% in the 8mg group and 34.0% in the 2mg group. At week 44, the rates were 39.6% and 28.3%, respectively. Also at week 44, mean change in central retinal thickness was -159.4µm in the 8mg group and -137.2µm in the 2mg group, while mean change in best-corrected visual acuity was +7.9 vs. +5.1 letter for the 8mg and 2mg group.
Although the study authors point out that aflibercept 8mg did not achieve its primary efficacy end point at week 16, the anatomic and visual improvements seen at week 44 indicate potential better therapeutic effects over the 2mg dose. In their JAMA Ophthalmology paper, they wrote that they believe “these findings support further evaluation of aflibercept, 8mg, in pivotal trials of exudative retinal diseases including nAMD and diabetic macular edema.”1
The authors then point out that although a small percentage of the 8mg group did receive additional treatment compared with the 2mg group, there was only a small difference in mean number of injections between the groups.
Even further, the authors relay that the almost eight-letter gain by week 44 in the 8mg group is similar to previous Phase III trials of approved anti-VEGF agents with dosing regimens of every four and every eight weeks. Some study trials have reported similar added therapeutic benefits from higher molar anti-VEGF treatments, notably ranibizumab 0.5mg over 0.3mg, ranibizumab 2mg over 0.5mg and brolucizimab 6mg over 3mg.
The researchers concluded by mentioning that their results are supported by recent release of 48-week primary endpoint data from the ongoing 3 PULSAR trial in nAMD, which found 8mg aflibercept injection every 12 or 16 weeks after three initial monthly injections saw noninferior visual gains and greater proportions of participants with dryness in the center subfield at weeks 16 and 48 compared with 2mg.
In an invited commentary also published by JAMA Ophthalmology, the point is brought up that “although trends can be valuable to help guide potential future studies, it is important not to overstate trends when applying them to clinical management, and especially when they were not preplanned primary or secondary endpoints.”2
In addition to the potential limitation of small sample size, the single-masked nature of the study should be kept in mind. Although this could put the study at risk for investigator bias, average injection number was similar in both groups. What was an issue was that the study “does not inform us if 8mg aflibercept would reduce treatment burden compared with 2mg aflibercept because the dosing schedule was fixed,” the commentary noted. The other issue is that the primary endpoint of fluid absence was not met at a level of statistical significance, leaving the question of how much is gained from higher-dose aflibercept compared with 2mg that has been used for over a decade.
Despite these valid questions raised, the commentary author also believes this study serves as a basis for the PULSAR study and PHOTON study focused on this intervention’s use in diabetic macular edema. Both the study and commentary authors agree that this valuable research will put into perspective some similar upcoming study results.
1. Wykoff CC, Brown DM, Reed K, et al. Effect of high-dose intravitreal aflibercept, 8mg, in patients with neovascular age-related macular degeneration: the Phase 2 CANDELA randomized clinical trial. JAMA Ophthalmol. August 3, 2023. [Epub ahead of print]. 2. Jhaveri C. The CANDELA Study—trends and end points. JAMA Ophthalmol. August 3, 2023. [Epub ahead of print]. |

