 |
Several years ago, a 66-year-old highly myopic white female presented as a new patient to the office with an interesting past social history. Her complaints centered around a gradual decrease in visual acuity O.U. at both distance and near.
Her last eye examination had been six years earlier. Her family ocular history was remarkable only for a maternal grandmother with glaucoma. She was taking no medications and reported allergies to Demerol (meperidine HCl, Sanofi-Synthelabo) and codeine.
Her family and social history as a young girl were quite unique. The patient was born in 1925 in Eastern Europe. The patient and her family were displaced several times in the political upheaval that followed WWI. With the outbreak of WWII, her main occupation was her daily survival. From her reports, food was scarce, and the constant stress of war wreaked havoc on her health and that of her family. She was, in essence, a war-torn civilian.
Diagnostic Data
At her initial visit, her best-corrected visual acuity was 20/30 O.D. and 20/40 O.S. through -6.76 -3.50 x 035 O.D. and -8.25 -2.50 x 160 O.S. Pupils were equal, round and reactive to light and accommodation, with no afferent papillary defect. However, the pupils did react slowly.
A slit lamp examination of her anterior segments was unremarkable. Applanation tensions were 18mm Hg O.U. Through dilated pupils, her crystalline lenses were clear O.U. Her cup-to-disc ratio was 0.25 x 0.25 O.U., with extremely pale neuroretinal rims bilaterally. The macular evaluations were normal O.U. Vessels were moderately attenuated.
Her peripheral retinal exam demonstrated marked pavingstone degeneration O.U., scattered areas of microcystoid degeneration O.U., and 360 degrees O.U. of dense reticular degeneration.
Threshold field studies demonstrated a mixed series of defects consisting of split fixation, cecocentral and paracentral defects; no defects respected the vertical hemianopic line.
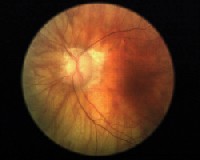 |
| Left eye of the patient: Note the pale atrophic neuroretinal rim with advanced glaucomatous cupping. |
She had her eyes examined only three or four times earlier in her life, and reported to me that none of the previous eye doctors mentioned anything unusual about her eyesin particular, the optic atrophy. Not knowing if the optic atrophy was recent or of long-standing duration, I ordered a baseline MRI, which was normal in all aspects. I also asked the patient to make an appointment with a primary care physician, which she didnt have. Subsequent evaluation by the PCP was unremarkable.
I saw the patient several times (albeit sporadic and inconsistent visits) over the next 10 years. The optic atrophy had remained stable, but her cup-to-disc ratio had increased to 0.85 x 0.85, with the majority of the increase in C/D ratio occurring in the last three years. IOPs remained in the mid-teens on each visit. She also began to develop cataracts O.U.
Her threshold visual fields over the last 2.5 years have deteriorated, attributable to the development of glaucomatous optic atrophy, the progression of the cataracts, and the pre-existing visual field abnormalities secondary to the optic atrophy.
Discussion
How do we know that she is developing glaucoma, and not undergoing progressive optic nerve changes associated with the long-standing optic atrophy? And why did her optic nerves undergo a significant amount of deterioration in a relatively short time in the presence of normal IOPs?
In general, the answer lies in the changes to the structure of the optic nerve that occur in glaucoma vs. the changes that occur in conditions that lead to optic atrophy. Numerous conditions may lead to optic atrophy, such as demyelinating disease, compressive disease, trauma, congenital abnormalities and developmental abnormalities, to name a few.
But what these conditions have in common is the lasting effect on the optic nerve: optic nerve pallor. Bear in mind that in classic optic atrophy, the neuroretinal rim remains intact (insofar as amount and volume); it loses its pink appearance due to the loss of fine papillary vasculature (thus the pallor). The neuroretinal rim, made up of the axons on retinal ganglion cells, remains in place, and does not generally undergo loss of volume (cupping). The loss of the fine vascular supply to the optic nerve headresulting in the pale appearanceoccurs as a result of the death of the ganglion cells, rather than vice versa.
Contrast that to progressive glaucomatous optic nerve damage. In cases of progressing optic cupping due to glaucoma, the neuroretinal rim itself becomes thinned over time due to actual physical loss of ganglion cells. The neuroretinal rim area essentially decreases as tissue volume is lost and, conversely, the cup increases. However, as glaucoma progresses, the remaining neuroretinal rim will retain a fine blood supply, leading to the characteristic thinned but plush neuroretinal rim.
Of course, in advanced glaucomatous damage, what minimal neuroretinal rim that remains has a proportionately minimal blood supply, resulting in a pale appearing optic disc. This paleness is not the pallor we associate with optic atrophy; it is simply the increasing visibility of the neural canal through the sclera and lamina.
In a review of multiple literature sources in September 2001, researchers at the University of Iowa argue that there is a preponderance of evidence implicating blood flowvascular insufficiency, in particularas a significant risk factor in both the development of glaucoma and ischemic optic neuropathy.1
As a result of this and other research, it is now common to describe the changes of a glaucomatous optic nerve as glaucomatous optic neuropathy. These researchers showed that in biopsy-positive giant cell arteritis-induced anterior ischemic optic neuropathy, the long-term effects to the optic nerve are decreased neuroretinal rim area and increased optic nerve cupping. This is the same pattern of damage as is seen in progressive glaucomatous nerve damage, thereby implicating a vascular overlap of the two disease processes.
The best way to evaluate patients such as ours is to closely examine the contours of the optic nerve. A detailed stereoscopic view of the optic nerves through dilated pupils is essential to evaluate the characteristics of the neuroretinal rim. Look specifically for loss of rim tissue, especially if the rim is already atrophically pale.
Secondarily, stereo optic nerve images are extremely important in monitoring change over time. In our office, we use the Synemed EyeScape digital imaging system, which has a software tool that displays a series of stereoscopic images of the optic nerve that can be morphed into one another over time, thereby highlighting subtle changes to the neuroretinal rim. In addition, the HRT II and the GDx VCC are valuable tools in quantifying changes to the optic nerve and perioptic RNFL.
Over the past several years, our patient has developed a series of cardiovascular conditions that ultimately affect peripheral perfusion. Chronic obstructive pulmonary disease (COPD), atrial fibrillation, atherosclerosis, hyperlipidemia, hyper- cholesterolemia and hypertension are but a few of the conditions that become more prevalent as our patient population ages. In her case, these additional systemic ailments may very well have played a role in further compromising a compromised disc, and facilitated her conversion to pressure-independent glaucomatous optic neuropathy.
Ultimately, the patient underwent bilateral trabeculectomies with two goals in mind. Cessation of progressive field loss by lowering IOP is the first indication for trabeculectomies. Secondarily, the trabeculectomies will help increase blood flow to the optic nerves, as evidenced by a recent study.2
1. Hayreh SS, Jonas JB. Optic disc morphology after arteritic anterior ischemic optic neuropathy. Ophthalmology 2001 Sep;108(9):1586-94.
2. Berisha F, Schmetterer K, Vass C, et al. Effect of trabeculectomy on ocular blood flow. Br J Ophthalmol 2005 Feb;89(2):185-8.

