 A 73-year-old Hispanic male presented with extremely rapid vision loss in his right eye that began the previous evening. The left eye seemed unaffected. He reported undergoing a comprehensive eye exam about two years ago, and said that his vision “was good” at that time. His medical history was significant for hypertension and type 2 diabetes, and he was properly medicated for both.
A 73-year-old Hispanic male presented with extremely rapid vision loss in his right eye that began the previous evening. The left eye seemed unaffected. He reported undergoing a comprehensive eye exam about two years ago, and said that his vision “was good” at that time. His medical history was significant for hypertension and type 2 diabetes, and he was properly medicated for both.
On examination, his visual acuity measured 6/200 O.D. and 20/25 O.S. Extraocular motility testing was normal. On confrontation visual field testing, he was only able to count fingers nasally O.D. The left eye, however, was full to careful finger counting. His pupils were equally round and reactive, with a 1+ afferent defect O.D. His anterior segment exam was unremarkable.
Dilated fundus exam showed small cups with good rim coloration and perfusion O.U. The posterior pole of the right eye showed obvious changes (figure 1). The left macula also exhibited some retinal pigment epithelium (RPE) changes (figure 2). The retinal vessels and periphery of the left eye were normal. We also ordered an optical coherence tomography scan (figure 3).
Take the Retina Quiz
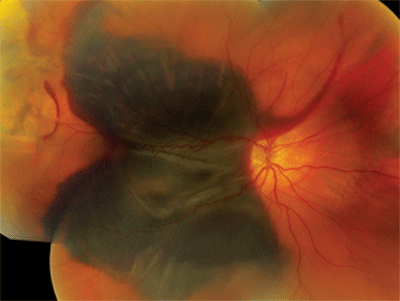
1. The right eye of our patient exhibits a massive hemorrhage.
1. What do the changes seen in the right posterior pole represent?a. Choroidal mass.
b. Choroidal detachments.
c. Hemorrhagic detachment of the RPE and sensory retina.
d. Preretinal hemorrhage.2. What additional testing would be most helpful to facilitate a diagnosis for this patient?
a. Fluorescein angiogram.
b. Indocyanine green angiography.
c. Ultrasound.
d. Electroretinogram.3. What is the likely etiology?
a. Aneurysmal choroidal vessels.
b. Choroidal neovascular
membrane.
c. Retinal neovascularization.
d. Mass that is penetrating Bruch’s membrane.4. What is the likely underlying cause of the etiology in question?
a. Choroidal melanoma.
b. Macroarterial aneurysm.
c. Wet macular degeneration.
d. Polypoidal choroidal vasculopathy (PCV).5. How should this patient be managed?
a. Observation.
b. Intravitreal Avastin (bevacizumab, Genentech) injection.
c. Surgical drainage of the subretinal hemorrhage with tissue plasminogen activator (tPA).
d. Laser.
For answers, see below.
Discussion
Our patient had a massive subretinal hemorrhage in his right eye. Additionally, he had detachments of the RPE and sensory retina. The fundus photo and OCT scan reveal the presence of lobulated macular elevations that were located both superiorly and inferiorly. Also, there were folds within the retina and macula that extended temporally as well as beyond the arcades.
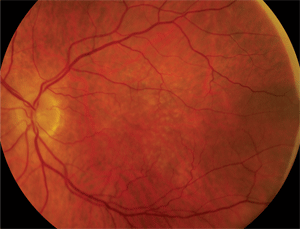
2. Here is a 2x-magnified view of our patient’s left eye.
The big question: What was the etiology of the hemorrhage? There are a number of causes for massive subretinal hemorrhages in the macula, including trauma, retinal arterial macroaneurysm (RAM), PCV and a choroidal mass breaking through Bruch’s membrane. The problem with such large hemorrhages is that it’s almost impossible to determine the cause by examination alone. However, there are some clues that can help narrow down the possibilities.
In this case, we ruled out trauma immediately because the patient denied being hit or having any accidents. Then, we noted that the retinal arteries were clearly visible and showed no evidence of RAM. Examination of the left macula revealed obvious drusen and RPE mottling. That observation alone was extremely important because the presence of drusen in the fellow eye––combined with the patient’s age––placed choroidal neovascularization secondary to AMD high on the list of differential diagnoses. But first, we needed to rule out the presence of a choroidal mass. So, we ordered a B-scan ultrasound, which showed no choroidal mass. PCV still could not be ruled out; however, the condition usually is seen in slightly younger patients, often of African or Middle Eastern descent.
Ultimately, wet AMD seemed to be the most likely underlying cause. So, the next question: What do we do about it? The prognosis for visual recovery from such a massive hemorrhage isn’t good, especially when it’s attributed to AMD. In one study of 60 eyes with subretinal hemorrhage secondary to AMD, 80% had a final visual acuity of 20/1250 or worse.1,2 Several factors account for this finding. The blood itself can be very toxic to the photoreceptors and the longer it’s present, the more destructive it is. In addition, the subsequent clot formation can have a detrimental effect.Finally, underlying retinal and RPE dysfunction caused by the disease can result in vision loss. For this reason, most retina specialists advocate surgical intervention, including removal of the hemorrhage, within the first few days of bleeding.
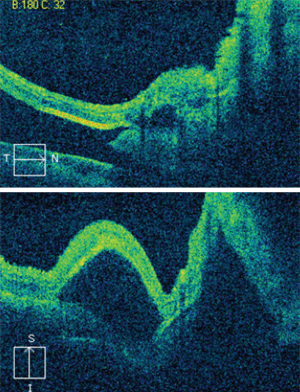
3. The horizontal (top) and vertical OCT scans through our patient’s right macula. What do you notice?
Indeed, our patient was taken to the operating room the next day for pars plana vitrectomy, air fluid exchange and surgical removal of the hemorrhage. Additionally, tPA was injected into the subretinal space at the time of the surgery. tPA is a thrombolytic anti-clot forming agent that has become the mainstay in treating large retinal and subretinal hemorrhages. The agent permits easier removal of the blood as well as alleviates any tractional components that develop secondary to blood clotting.
We saw our patient one day after the surgery. At that time, we noted some residual vitreous hemorrhage and an air bubble. He received an intravitreal Avastin injection and was asked to return in two weeks for further follow-up. The patient has yet to return. Unfortunately, the prognosis for visual recovery is poor.
1. Lee HC, Holekamp NM. The surgical management of submacular hemorrhage. In: Ryan SJ (ed.). Retina, Vol. III––Surgical Retina, 4th ed. St. Louis: Mosby; 2006:2555-60.
2. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213(2):97-102.
Answers to the Quiz
1. c
2. c
3. b
4. c
5. c

