In 2010, 2.72 million Americans had glaucoma—1.9% of the US population older than 40 at the time.1 This number is projected to increase to four million by 2030 and 6.3 million by 2050.1 However, open-angle glaucoma may be over-diagnosed in as much as 50% to 60% of our patients.2-5 While studies show that patients with a family history of glaucoma, are older in age or who are African American are more likely to be over-diagnosed, other clinical features often lead to over-diagnosis as well, such as larger nerves (and resultant larger cupping) and intraocular pressure (IOP) above 21mm Hg.2-5 In addition, glaucoma may also be misdiagnosed when the patient’s history is not taken into proper context or the neuroretinal rim is not properly evaluated.
These cases highlight the challenges of differentiating glaucoma from ocular hypertension or other conditions—and the importance of a thorough medical history and clinical exam.
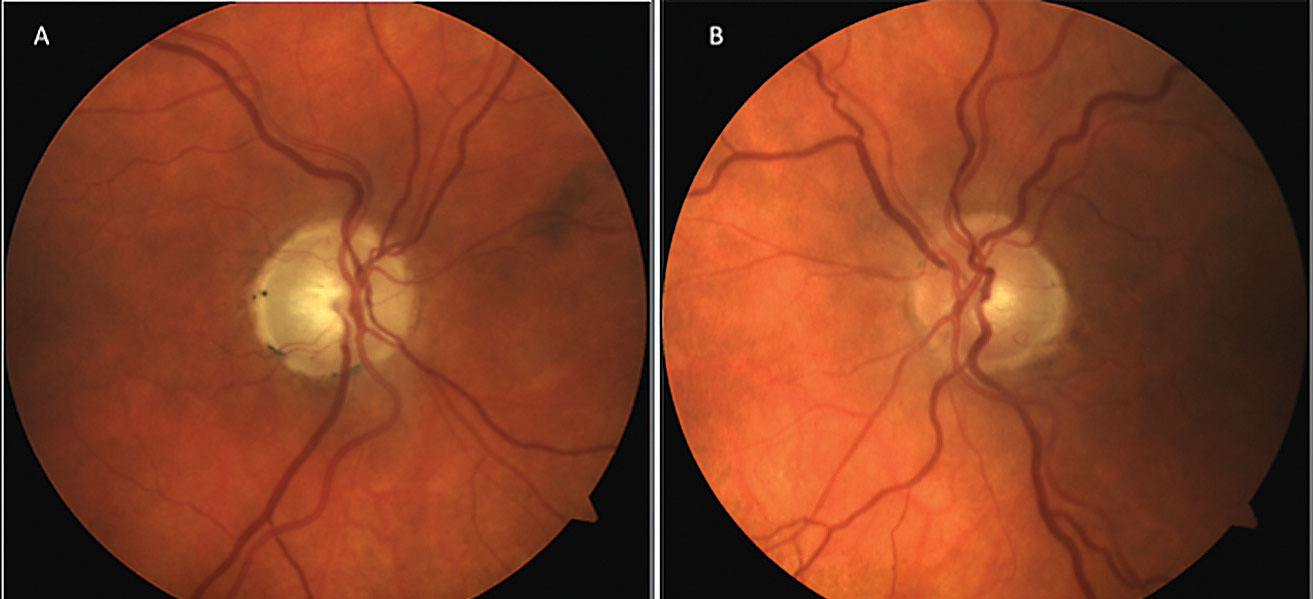 |
| Fig. 1. The patient’s optic nerve fundus photo shows evident neuroretinal rim pallor OD (A) and early glaucomatous cupping OS (B). Click image to enlarge. |
Case 1: Trauma Drama
A 58-year-old male was referred for a glaucoma evaluation due to enlarged cupping OU (Figure 1). His review of systems and personal ocular history was unremarkable other than a history of significant trauma to the right side of his head years ago. His best-corrected visual acuity (BCVA) was 20/50+2 OD and 20/20 OS with grade 1 relative afferent pupillary defect OD. His IOPs were in the mid to upper teens with average thickness pachymetry measurements. Gonioscopy showed that the ciliary body was visible in all four quadrants with lightly pigmented trabecular meshwork (TM) OU, flat iris approach and no obvious angle recession OD.
Given his history of trauma and preliminary test results, we diagnosed him with non-glaucomatous traumatic optic neuropathy OD and early glaucomatous optic neuropathy (GON) OS.
 |
| Fig. 2. This optic nerve fundus photo reveals significant focal thinning at 7 o’clock OD (A) and moderate generalized cupping without focal rim thinning OS (B). Click image to enlarge. |
Discussion. Histologically, GON is an ascending or anterograde neuropathy (damage occurring from retina to brain), which is characterized by retinal ganglion cell loss that extends upwards along the axons, resulting in neuroretinal rim loss. Non-glaucomatous optic neuropathy, however, is a descending or retrograde neuropathy (damage occurring from brain to retina) resulting from more than a dozen retinal, optic nerve and neurological disorders that ultimately lead to a loss of capillary perfusion and neuroretinal rim atrophy—with pallor that classically extends beyond any optic nerve cupping (Table 1).6
Clinically, GON is usually associated with older age, elevated IOPs, thinner pachymetry, visual acuity better than 20/40, greater cup-to-disc ratio with vertical elongation (especially focal neuroretinal rim loss), minimal, if any, neuroretinal rim pallor, greater frequency of parapapillary atrophy (especially beta zone) and optic disc hemorrhages in the inferior temporal and superior temporal sectors that are associated with retinal nerve fiber layer defects.6,7 GON is also more likely to be associated with a confirmed family history of glaucoma and with visual field defects that tend to respect the horizontal meridian and have a relatively strong correlation with the optic nerve appearance.6
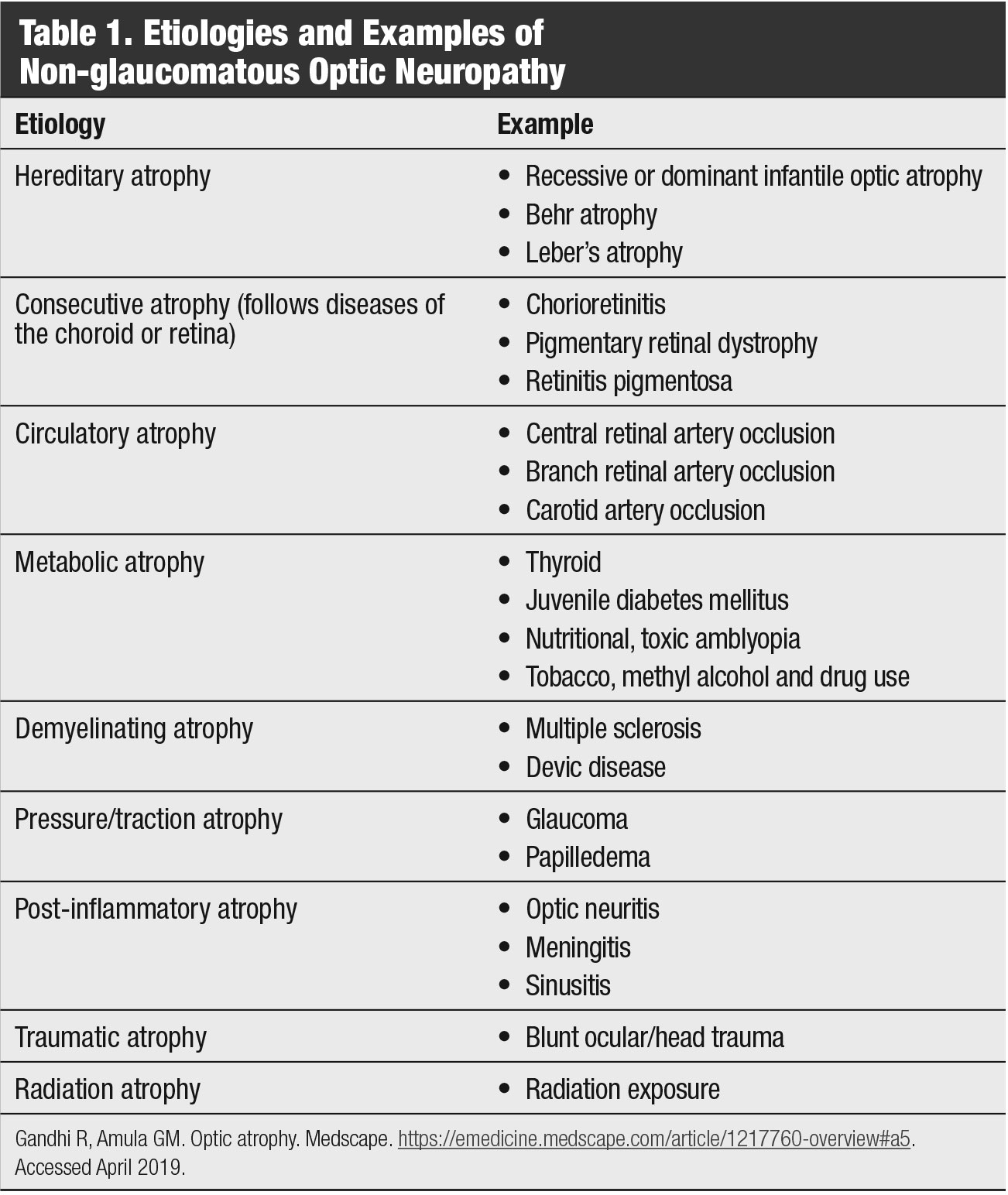 |
| Table 1. Etiologies and Examples of Non-glaucomatous Optic Neuropathy. Click table to enlarge. |
Non-glaucomatous optic neuropathy is usually not associated with neuroretinal rim loss—just pallor—and complete rim loss is never present.6 Furthermore, non-GON does not commonly have significant parapapillary atrophy and, if it does, it rarely progresses. Additionally, and unlike GON, non-GON is usually associated with younger age (younger than 50), visual acuity worse than 20/40 and visual field defects that respect more the vertical meridian and are less likely to correlate with the optic nerve appearance.6,8-10 Of particular clinical significance, non-GON GCC loss occurs prior to RNFL loss.
Despite these distinctions, some similarities between GON and non-GON can potentially lead to misdiagnosis. Both conditions can sometimes share the following morphological features: decreased retinal arteriole diameter with focal arteriole narrowing, reduced retinal nerve fiber layer (RNFL) visibility, localized RNFL defects and enlargement/deepening of the optic cup with correlating decreased neuroretinal rim thickness.7-10
Aside from the ever-important patient history, the most valuable qualitative variables for differentiating GON from non-GON is the neuroretinal rim thickness/perfusion appearance, the strength/appearance of the correlating visual field defects and the presence of associated glaucomatous disc hemorrhages. An accurate diagnosis is more likely to lead to a proper work-up (if indicated) and more appropriate treatment options.
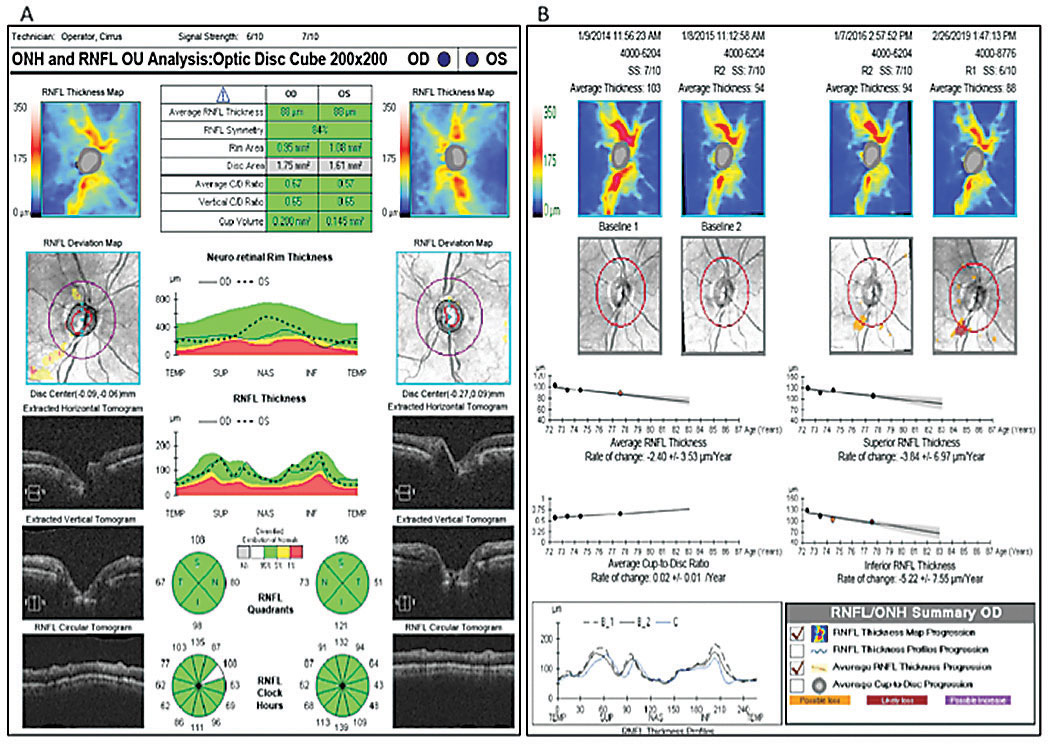 |
| Fig. 3. OCT RNFL analysis shows focal inferotemporal RNFL loss OD (A), while RNFL guided progression analysis shows steep negative slopes of the inferior RNFL thickness OD, more so than the average and superior thicknesses (B). Click image to enlarge. |
Case 2: Tensions Running Low
A 77-year-old white male returned to the clinic 32 months after being lost to follow-up for glaucoma suspicion due to asymmetric cupping OS>OD. His ocular history was otherwise unremarkable, and he had no new complaints. His medical history included chronic lymphocytic leukemia, hypertension, hyperlipidemia, benign prostatic hyperplasia and hypothyroidism for which he was taking lisinopril, simvastatin, tamsulosin and levothyroxine. BCVAs were 20/25 OD and 20/20 OS with hyperopic and astigmatic correction. Pupil, extra-ocular muscle and confrontation field tests were all normal.
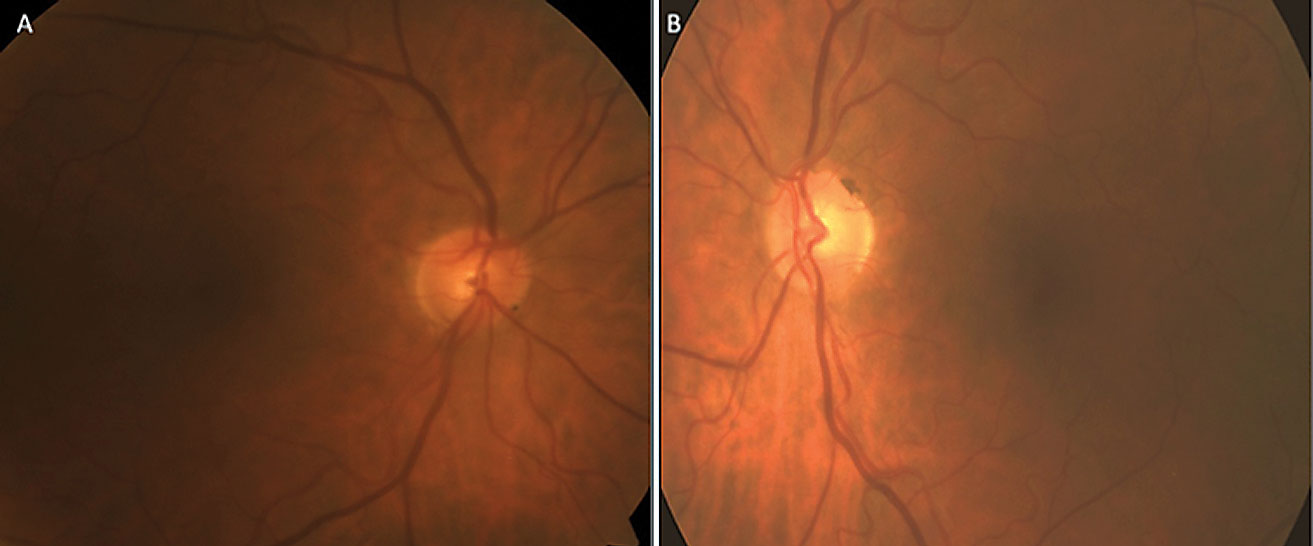
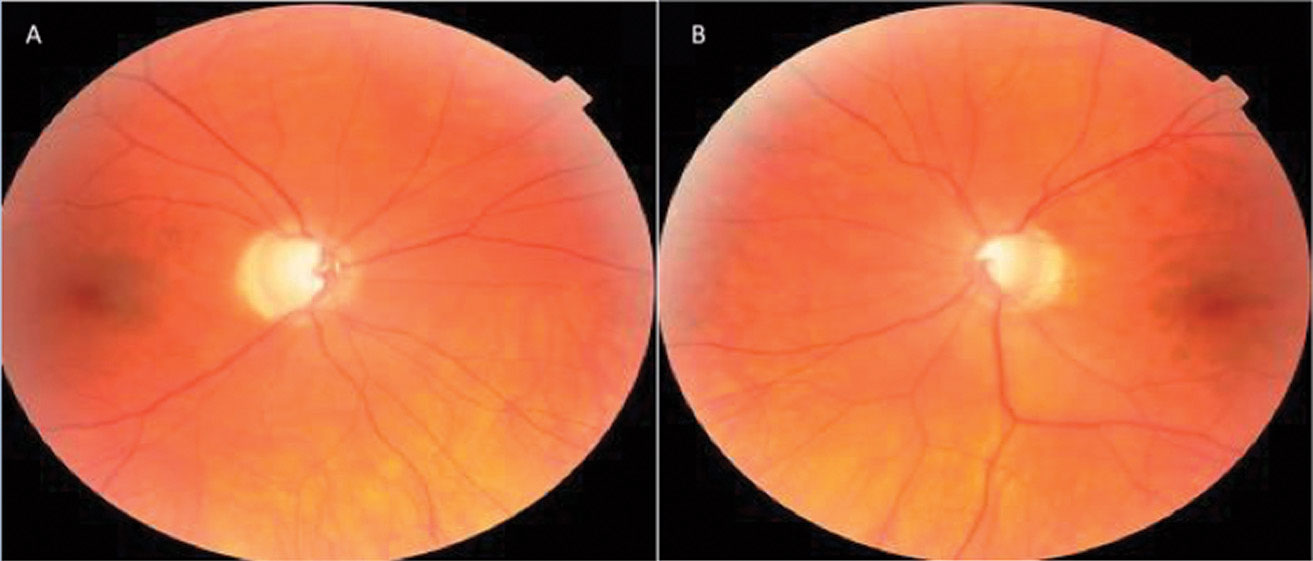
His anterior segments were normal with deep chambers. There was moderate nuclear sclerosis OD>OS. IOPs were 20mm Hg OU. On prior exams, his untreated IOP ranged from 17mm Hg to 21mm Hg OD and 16mm Hg to 20mm Hg OS with an average of 19mm Hg OU. His central corneal thicknesses were 553µm OD and 541µm OS. Dilated fundus exam was concerning for vertical cupping and focal inferotemporal neuroretinal rim thinning OS. Cup-to-disc ratios were 0.6x0.4 OD and 0.55x0.55 OS (Figure 2).
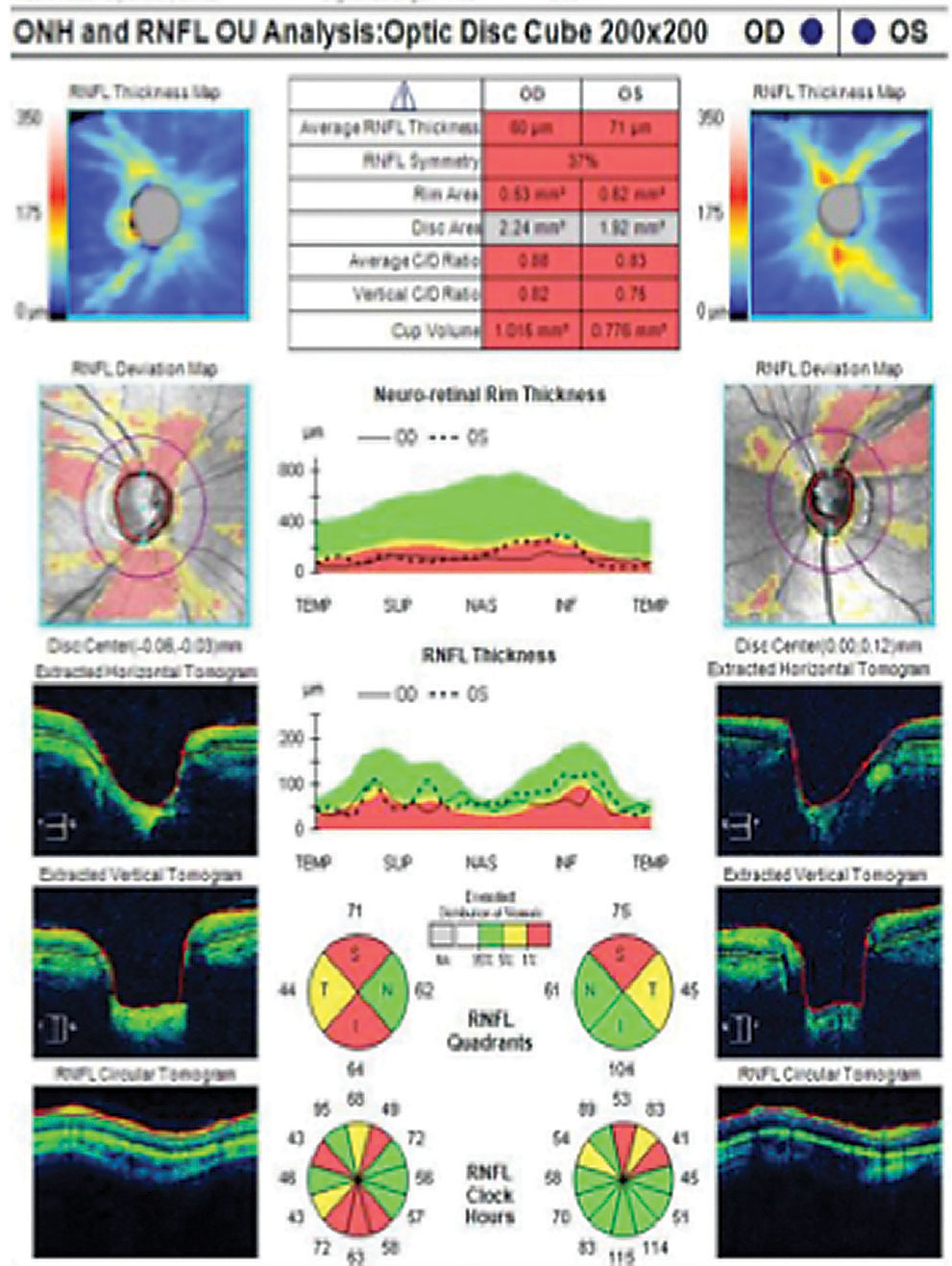
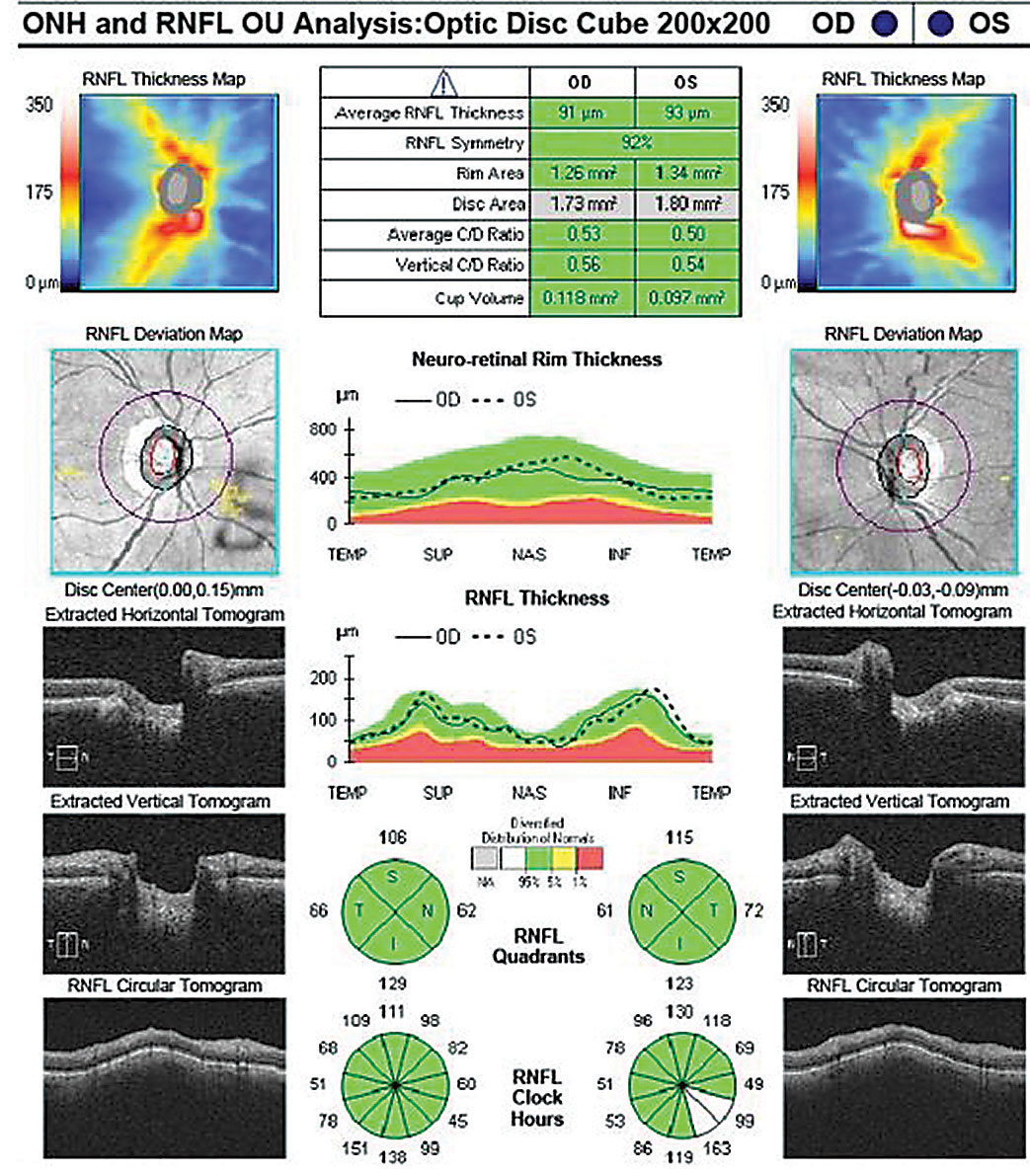
OCT RNFL analysis demonstrated normal average, quadrant and sector thicknesses (Figure 3A). However, on closer inspection, the RNFL TSNIT graph showed significant focal thinning inferotemporal OD. Furthermore, the color-coded RNFL deviation map and the qualitative review of the RNFL circular tomogram both showed thinning.
 |
| Fig. 4. This patient’s optic nerve fundus photo indicates evidence of glaucomatous thinning and cupping, and papapapillary atrophy OD (A) and OS (B). Click image to enlarge. |
Research shows thinning of the RNFL at the 7 o’clock sector OD and 5 o’clock sector OS is the most useful discriminant between normal and mild glaucoma among all optic nerve head (ONH) and RNFL parameters included in a single OCT 200x200 optic disc cube.11 Although this case shows 84% symmetry as normal (95% of normals in the normative database range from 76% to 95% for a 69-year-old, for example), we did note the inferior quadrant and inferotemporal 7 o’clock wedge sector was 23µm thinner OD than OS.12
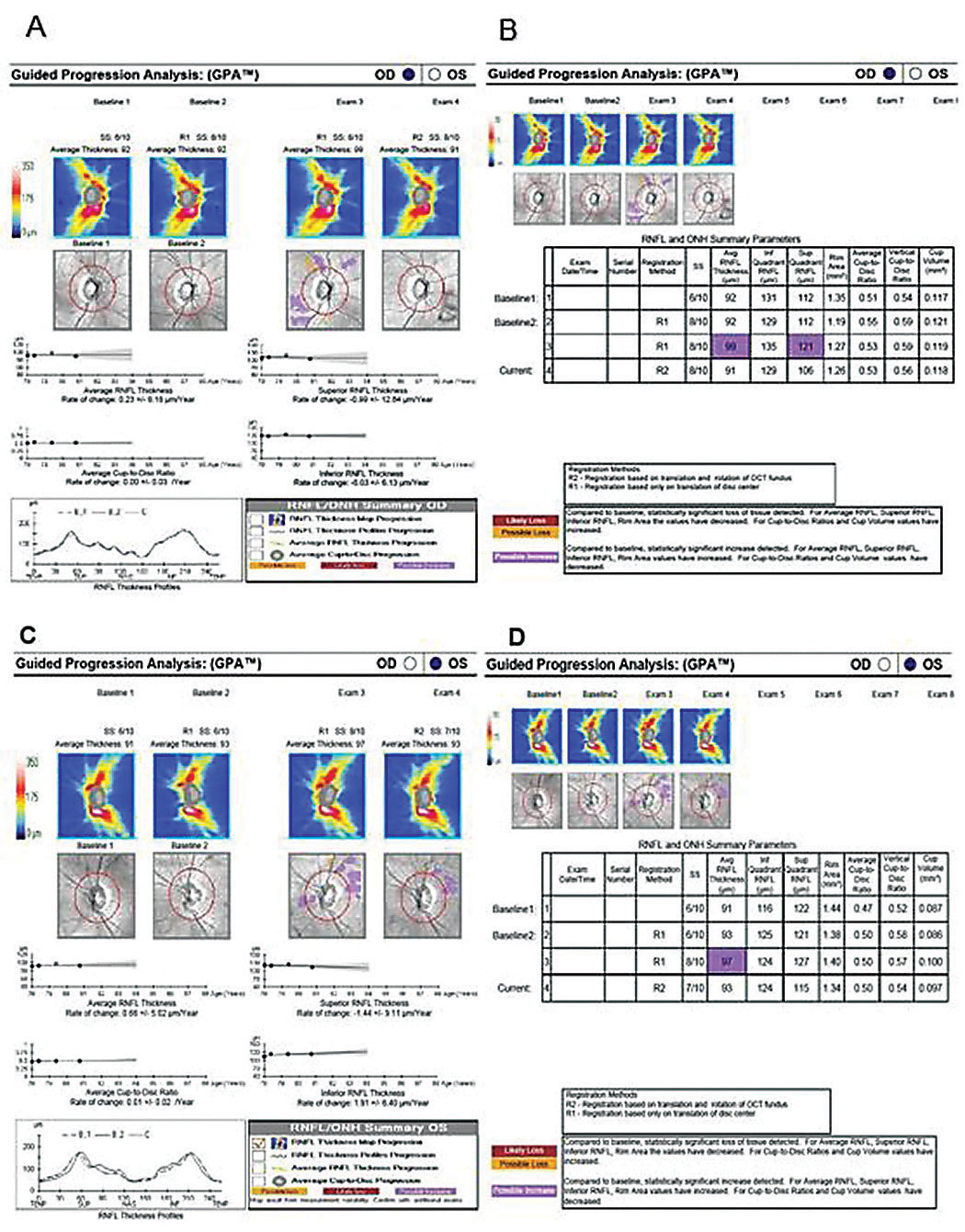
The absence of red or yellow color flag on a single OCT RNFL analysis may lessen the concern for GON, or worse, raise doubt about the ONH assessment on exam. Glaucoma in the context of a normal or “all green” OCT RNFL analysis constitutes a false negative, often referred to as “green disease.”14 In this case, prior scans allowed for guided progression analysis (Figure 3B). The report showed new, statistically significant reduction in the average RNFL thickness and continued thinning of the inferior thickness OD. In one study, repeatable loss of ≥4µm was considered significant when comparing scans of adequate signal strength, ≥6µm or 7µm.11,13
Given these findings, the patient was diagnosed with low-tension, open-angle glaucoma of indeterminate stage OD, and he remained a suspect OS.
 |
| Fig. 5. OCT imaging shows advanced thinning on the thickness and deviation maps, which correlates with the circular RNFL tomogram. Click image to enlarge. |
Discussion. While most consider increased IOP a hallmark of glaucoma, large population-based studies show as many as 32% of open-angle glaucoma patients have IOP less than 21mm Hg, considered low-tension glaucoma.14 OCT structural evaluation of the ONH is a valuable tool to help assess low-tension glaucoma and can be more sensitive than standard automated perimetry in the early disease state.13,15 Thus, this technology is now a standard component of the evaluation to aid in the early detection of all types of GON.13,15
Although the OCT normative database is a useful benchmark, it does have limitations. Clinicians should carefully interpret scans beyond the red, yellow and green designations. Clinicians can recognize green disease by scrutinizing the TSNIT graphs, qualitatively reviewing the peripapillary RNFL B-scans (while being mindful for segmentation errors) and using serial scans with progression analysis. In addition, inferotemporal RNFL thinning on OCT imaging is a key element in the detection of early or pre-perimetric glaucoma. Despite OCT’s firm foothold in the standard of care for diagnosing and monitoring GON, careful scrutiny of the neuroretinal rim on funduscopy is vital.
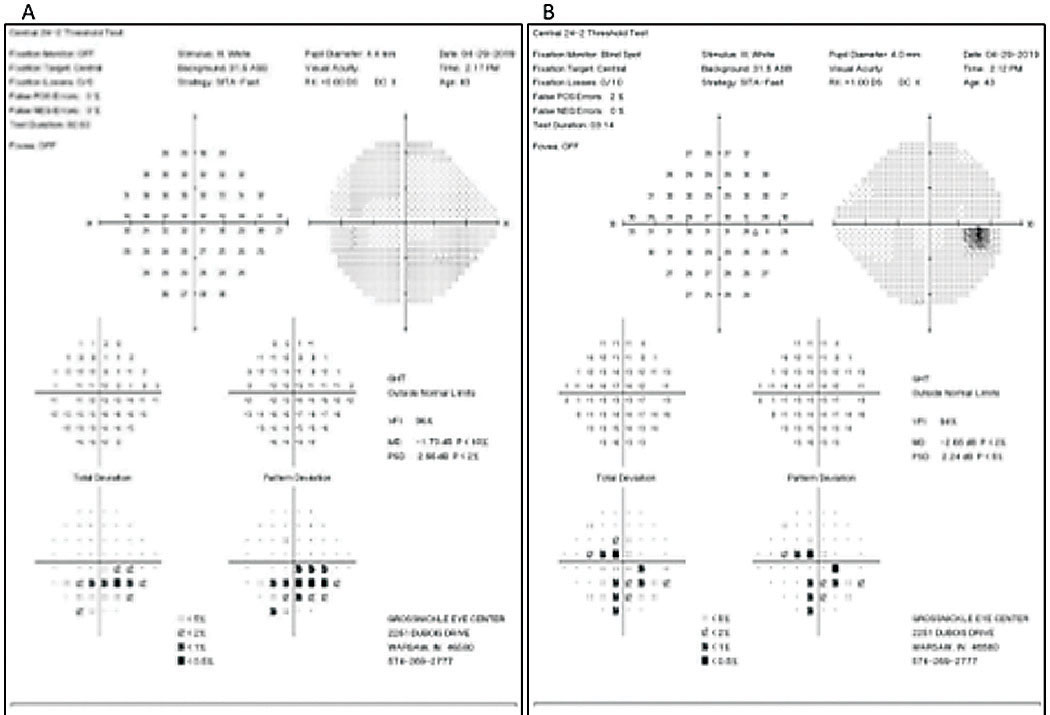 |
| Fig. 6. The patient’s SAP (24-2) reveals moderate inferior arcuate to fixation OS (A) and moderate inferior arcuate to fixation and superior central and paracentral defects OD (B). Click image to enlarge. |
Case 3: Pigments on the Loose
A patient in his late 30s was referred for a glaucoma evaluation with treatment-naïve elevated IOPs in the mid to low 30s and glaucomatous optic nerve appearance OU (Figure 4). His review of systems and ocular history was unremarkable other than a history of moderate- to high myopia (-7.50D OD, -8.50D OS) with a history of LASIK OU more than a decade ago. His BCVA was 20/20 OD and 20/20 OS with no relative afferent pupillary defect OU. Gonioscopy showed that the ciliary body was visible in all four quadrants with 3 to 4+ TM pigmentation, and slightly concave iris approach OU. The exam also showed multiple midperipheral, distinct transillumination defects and associated bilateral endothelial Krukenberg spindles OU. His preoperative pachymetry measurements were 574µm OD and 595µm OS.
Given the patient’s age, advanced neuroretinal rim thinning, OCT thinning and correlating visual field defects, he was diagnosed with pigmentary dispersion glaucoma (Figures 5 and 6).
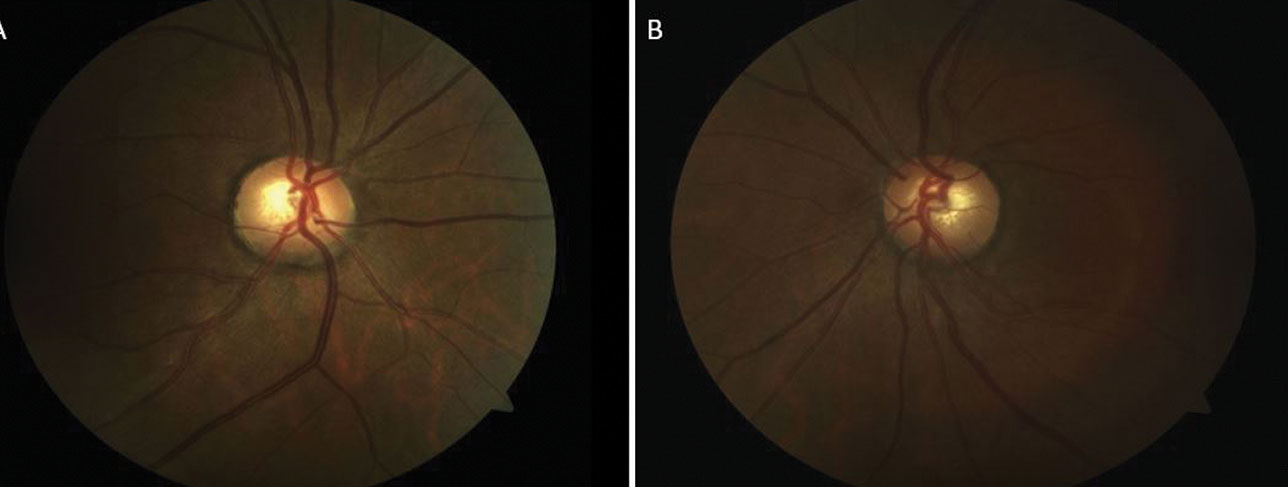 |
| Fig. 7. The patient’s optic nerve fundus photo shows a healthy rim and moderate cup OD (A) and OS (B). Click image to enlarge. |
Discussion. More than a dozen secondary forms of glaucoma exist, and research shows they account for about 22% of all cases of confirmed glaucoma or glaucoma suspect patients; 57% of these patients have IOPs above 30mm Hg (Table 2).16,17 These patients often have a positive history and ocular findings of trauma, surgery, neovascularization, inflammation or other abnormal findings that might explain the increased IOP.17 They require active monitoring and aggressive treatment due to their commonly advanced stage and younger age at presentation.17
This case focuses on one of the more common forms, pigmentary glaucoma, which is a potential endpoint for those with pigmentary dispersion syndrome. The syndrome is a clinical triad first classified as mid-peripheral transillumination iris defects, pigment deposits on the corneal endothelium (Krukenberg spindle) and heavy TM pigmentation.18,19 Reseaerch shows that the latter of these findings is what potentially leads to elevated IOPs and, if not properly treated, irreversible glaucomatous damage.20 Conversion to glaucoma is possible in up to 50% of those with pigmentary dispersion syndrome.21 Furthermore, one study reported the risk for developing pigmentary glaucoma from pigment dispersion syndrome was 10% at five years and 15% at 15 years. Those with an IOP greater than 21mm Hg at the initial examination also had an increased risk of conversion.22
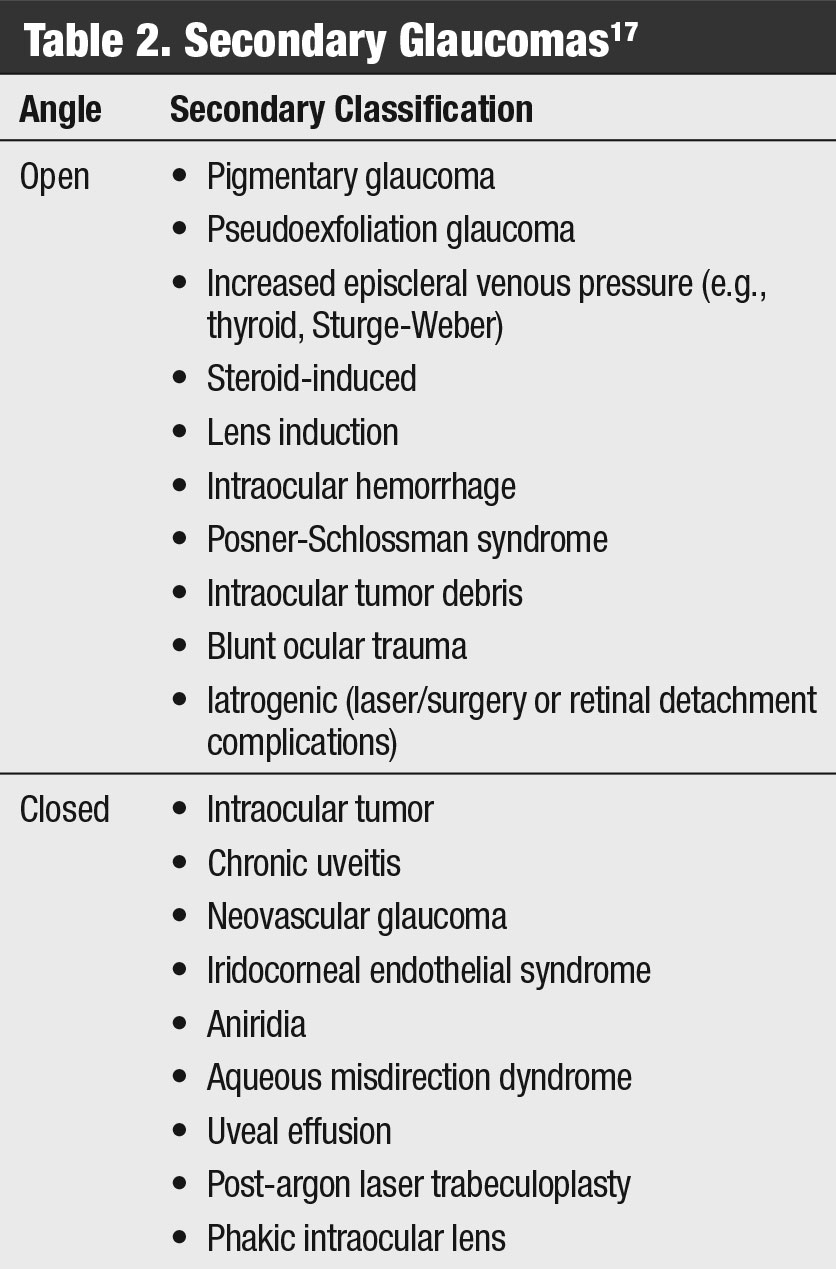 |
| Table 2. Secondary Glaucomas. Click table to enlarge. |
Younger, white, myopic males are most at risk for pigmentary glaucoma, and they are usually diagnosed in their 3rd or 4th decades of life.21 Fortunately, with a timely diagnosis, these patients usually respond well to topical treatment and selective laser trabeculoplasty.
One of the most important tools for differentiating between primary and secondary glaucoma is gonioscopy. This technique, along with a thorough patient history, leads to a more accurate diagnosis, a proper work-up (if indicated) and appropriate treatment options.
 |
| Fig. 8. This retinal nerve fiber layer OCT scan shows a normal TSNIT graph, normal quadrant and sectoral tomogram, and robust thickness maps OU. Click image to enlarge. |
Case 4: Debris Holding Up the Works
A 78-year-old Caucasian male reported to our clinic for a routine eye exam. He reported an ocular history of “atypical” cataracts, but did not elaborate. He was not taking any ocular medications, and his BCVA was 20/20 OD and OS. Pupils, extraocular muscles and confrontation field tests were all normal. His IOPs measured 28mm Hg OD and 30mm Hg OS.
Notable anterior segment findings included a white, fluffy material around the pupillary margin OU, no transillumation iris defects OU, and no corneal endothelial pigment OU. Gonioscopy showed an open angle 360°, increased TM pigmentation and Sampaolesi’s line inferiorly. This increased pigmentation in the TM resembled a “brown sugar” appearance. All other anterior segment findings were within normal limits.
His dilated fundus exam showed a “ground glass” appearance of the anterior lens capsule, and retro-illumination of the lens showed a “bull’s-eye” appearance. There was a clear vitreous, a macula that was flat with a mild epiretinal membrane, an arterial-venous ratio of 2/3 and a peripheral retina that was flat and intact with no pathology noted OU.
 |
| Fig. 9. RNFL guided progression analysis OD (A) and OS (C) shows stable RNFL thickness slopes. Furthermore, the RNFL and ONH parameters remain stable OD (B) and OS (D). Click image to enlarge. |
The optic nerves had a cup-to-disc ratio of 0.55x0.55 OD and OS, and were of average size and normal shape (Figure 7). There was no Drance hemorrhage, pallor, loss of perfusion or RNFL wedge defect OU. An RNFL OCT was within normal limits OD and OS (Figure 8). GCC was deferred due to epiretinal membrane artifacts. Pachymetry readings measured by OCT were 550µm OD and 548µm OS.
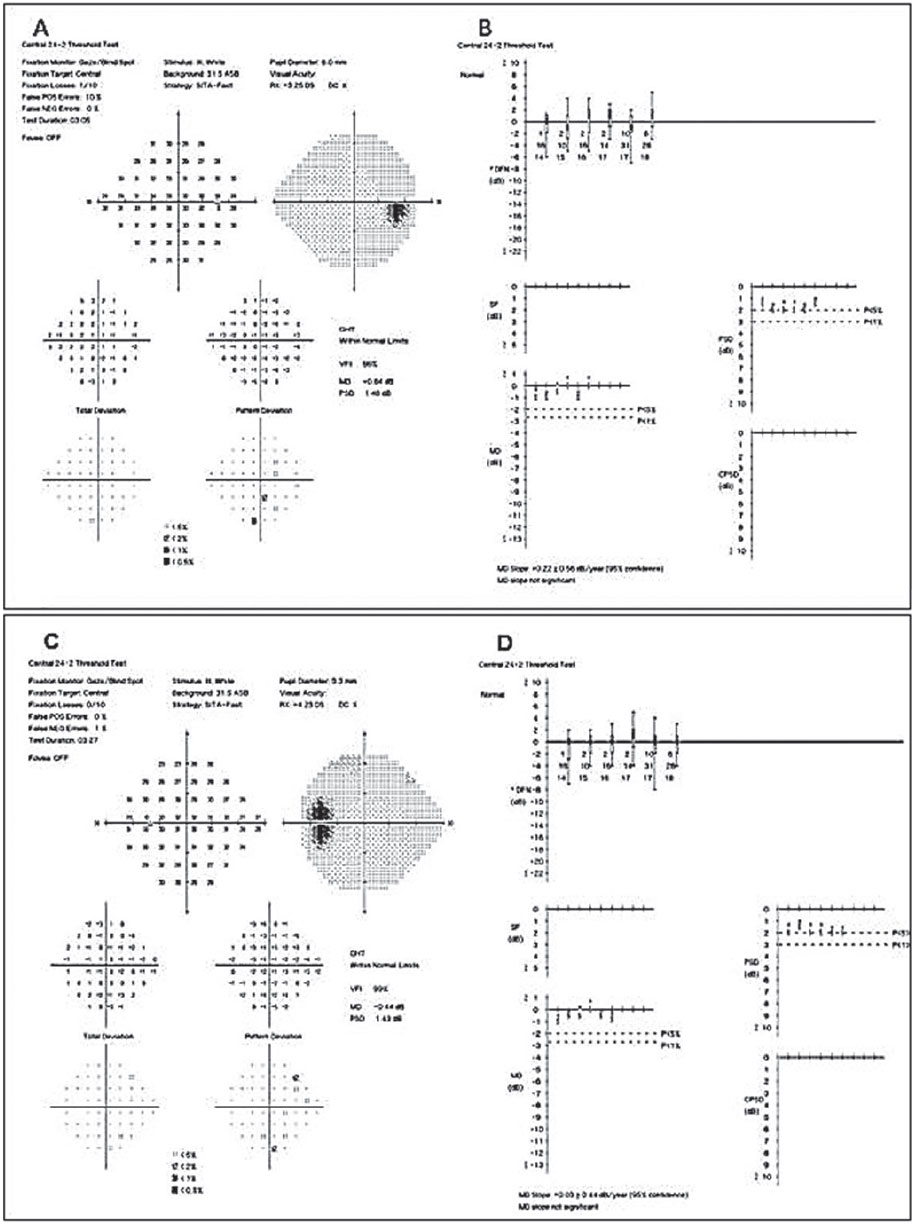
Based on these findings we diagnosed the patient with ocular hypertension and pseudoexfoliation (PXF) syndrome in both eyes. However, we did not initiate any topical hypotensive treatment at this visit, as we wanted to acquire additional IOP measurements first. Over a series of four office visits (a few months), the patient had an untreated IOP range of 26mm Hg to 32mm Hg OD and 28mm Hg to 33mm Hg OS. We then initiated topical hypotensive treatment with latanoprost QHS OU due to the aggressive nature of PXF and the increased risk PXF possess when IOP is above threshold. He returned six weeks later and his IOP measured 16mm Hg OU, and his SAP 24-2 visual fields were within normal limits (Figure 9).
We followed this patient with serial visual fields and OCT exams. The RNFL OCT and visual fields remained stable over several years as shown in the GPA analyses (Figure 10). He required no further intervention.
 |
| Fig. 10. The patient’s most current SAP (24-2) visual fields show OD (A) and OS (C) being within normal limits and without glaucomatous defects. GPA slope OD (B) and OS (D) are stable to baseline, and the MD slope is “not significant.” Click image to enlarge. |
Discussion. PXF is a systemic disorder characterized by progressive accumulation and granular deposition of abnormal extracellular pseudoexfoliative material in ocular tissues and many organs throughout the body. Deposits are composed of amyloid, laminin, collagen, elastic fibers and basement membrane.23
PXF is predominantly associated with those of northern European and Scandinavian descent, but can be found in all populations and races.24 Patients with PXF and IOPs above the normal threshold have double to triple the risk of developing glaucoma compared with those without PXF and IOP above the normal threshold.25,26
IOP elevation in PXF is caused by increased outflow resistance in the TM due to the blockage caused by pseudoexfoliative material. Exfoliation debris inherently occur and accumulate in the TM, which subsequently alters and damages the juxtacanalicular tissue beneath the inner wall of Schlemm’s canal, causing increased IOP.27,28 Pseudoexfoliation glaucoma (PXG) has a worse prognosis than primary open-angle glaucoma due to higher IOP levels, greater diurnal fluctuations and IOP spikes.27,29 Topical hypotensive therapies are generally effective in lowering IOP in PXG cases initially, but due to the condition’s refractory and recalcitrant nature, surgical options are usually needed.27
A patient’s IOP is only a small part of the overall ocular picture. High IOP doesn’t necessarily indicate glaucoma, and low IOP doesn’t rule it out, either. Only with a thorough medical history and clinical examination, augmented by diagnostic tools such as fundus photography, OCT and visual fields, can clinicians see the whole picture and provide an accurate diagnosis.
Drs. Brian Fisher and Johnson work at The Villages VA Outpatient Clinic, The Villages, Fla.
Dr. Lifferth is an optometrist at Grossnickle Eye Center in Indiana.
Dr. April Fisher is an optometrist at Ocala West VA Community Based Outpatient Clinic, Ocala, Fla.
1. National Eye Institute. Open-angle glaucoma defined. nei.nih.gov/eyedata/glaucoma. Accessed July 25, 2019. 2. Founti P, Coleman AL, Wilson MR, et al. Overdiagnosis of open-angle glaucoma in the general population: the Thessaloniki Eye study. Acta Ophthalmol. 2018;96(7):e859-64. 3. Nayak BK, Maskati QB, Parikh R. The unique problem of glaucoma: under-diagnosis and over-treatment. Indian J Ophthalmol. 2011;59(Suppl1):S1-S2. 4. Swanson, MW.Undiagnosed and overdiagnosed glaucoma in the United States. Invest Ophthalmol Vis Sci. 2012;53(14):6379. 5. Ahmad SS. Glaucoma suspects: A practical approach. Taiwan J Ophthalmol. 2018;8:74-8. 6. Zhang YX, Huang HB, Wei SH. Clinical characteristics of nonglaucomatous optic disc cupping. Exp Ther Med. 2014;7(4):995-99. 7. Jonas JB, Budde WM, Panda-Jones S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43(4):293-320. 8. Choudhari N, Neog A, Fudnawala V, George R. Cupped disc with normal intraocular pressure: the long road to avoid misdiagnosis. Indian J Ophthalmol. 2011;59(6):491-97. 9. Greenfield D, Siatkowski R, Glaser JS, et al. The cupped disc. Who needs neuroimaging? Ophthalmol. 1998;105(10):1866-74. 10. Greenfield D. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Sem Ophthalmol. 1999;14(2):95-108. 11. Mwanza J, Oakley JD, Budenz DL, et al. Ability of Cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmol. 2011;118(2):e241-48. 12. Carl Zeiss Meditec. Cirrus HD-OCT: How to read the Cirrus reports. www.zeiss.co.uk/content/dam/Meditec/gb/Chris/OCT Business Builder/PDF’s/1.pdf. Accessed April 23, 2019. 13. Sayed MS, Margolis M, Lee RK. Green disease in optical coherence tomography diagnosis of glaucoma. Curr Opin Ophthalmol. 2017;28(2):139-53. 14. Krupin T, Liebmann JM, Greenfield DS, et al. The Low-pressure Glaucoma Treatment Study (LoGTS). Ophthalmology. 2005;112(3):376-85. 15. Nguyen AT, Greenfield DS, Bhakta AS, et al. Detecting glaucoma progression using guided progression analysis with OCT and visual field assessment in eyes classified by international classification of disease severity codes. Ophthalmol Glaucoma. 2019;2(1):36-46. 16. European Glaucoma Society. Terminology and Guidelines for Glaucoma, 4th ed. Br J Ophthalmol. 2017;101(4):1-72. 17. Gadia R, Sihota R, Dada T, Gupta V. Current profile of secondary glaucomas. Indian J Ophthalmol. 2008;56(4):285-89. 18. Richter CU, Richardson TM, Grant WM. Pigmentary dispersion syndrome and pigmentary glaucoma: a prospective study of the natural history. Arch Ophthalmol. 1986;104(2):211-15. 19. Sugar HS, Barbour FA. Pigmentary glaucoma: a rare clinical entity. Am J Ophthalmol. 1949;32(1):90-2. 20. Greenstein VC, Seiple W, Liebmann J, Ritch R. Retinal pigment epithelial dysfunction in patients with pigmentary dispersion syndrome. Implications for the theory of pathogenesis. Arch Ophthalmol. 2001;119(9):1291-5. 21. Farrar SM, Shields MB, Miller KN, Stoup CM. Risk factors for the development and severity of glaucoma in the pigment dispersion syndrome. Am J Ophthalmol. 1989;108(3):223-9. 22. Siddiqui Y, Ten Hulzen RD, et al. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135(6):794-799. 23. Tekin K, Inanc M, Elgin U. Monitoring and management of patients with pseudoexfoliation syndrome: current prespectives. Clin Ophthalmol. 2019:(13):453-64. 24. Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45(4):265-315. 25. Topouzis F, Harris A, Wilson MR, et al. Increased likelihood of glaucoma at the same screening intraocular pressure in subjects with pseudoexfoliation: the Thessaloniki eye study. Am J Ophthalmol. 2009;148(4):606-13. 26. Grødum K, Heijl A, Bengtsson B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation. Ophthalmology. 2005;112(3):386-90. 27. Schlotzer-Schrehardt U, Naumann G. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141(5):921-37. 28. Gottanka J, Flügel-Koch C, Martus P. Correlation of pseudoexfoliative material and optic nerve damage in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 1997;38:2435-46. 29. Musch DC, Shimizu T, Niziol LM, et al. Clinical characteristics of newly diagnosed primary,pigmentary and pseudoexfoliative open-angle glaucoma in the Collaborative Initial Glaucoma Treatment study. Br J Ophthalmol. 2012;96(9):1180-84. |

