Bausch & Lombs ReNu with MoistureLoc contact lens solution is off the worldwide market, but investigators continue to look into the increased number of reported fungal infections among contact lens wearers.
As of May 18, 2006, there were 130 confirmed cases of Fusarium keratitis infection in the United States, the Centers for Disease Control and Prevention said in its May 26 Morbidity and Mortality Weekly Report. Of these cases, 125 patients reported wearing contact lenses, and 118 were able to identify which contact lens solution(s) they had used the month before onset of infection.
Specifically:
Seventy-five patients (64%) reported using only Bausch & Lombs ReNu with MoistureLoc.
Fourteen patients (12%) reported using MoistureLoc and another product.
Eight patients (7%) reported using an unspecified Bausch & Lomb solution.
Twenty-one patients (18%) reported using products other than MoistureLoc.
B&L voluntarily withdrew MoistureLoc from the U.S. market in April and last month announced that it would discontinue worldwide distribution.
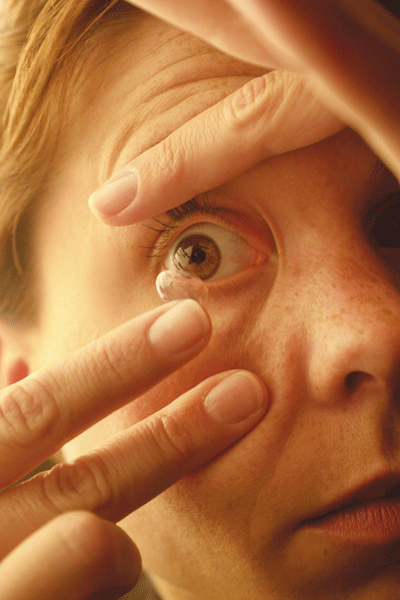 |
| Improper contact lens care very likely plays a role in the recent outbreak of Fusarium keratitis infections. |
In a report issued on May 15, FDA investigators listed 18 observations that it says deviate from good manufacturing practices. Among them:
B&L failed to notify the FDA of 35 serious injury reports of Fusarium keratitis relating to ReNu with MoistureLoc from Singapores Minister of Health in February 2006 and B&Ls subsequent removal of the product from Singapore and Hong Kong that same month.
The Greenville plant did not follow procedures to prevent contamination of equipment or the product by certain substances.
The plant did not define and document appropriate procedures for controlling environmental conditions.
Even so, the FDA points out that these observations are preliminary, and they do not appear to have contributed to the rise in Fusarium cases. There was no evidence found of product contamination at the Greenville facilities.
B&L denies that it failed to promptly report the unusual spike in Fusarium cases among contact lens wearers in Singapore to the FDA. Specifically, B&L says it discussed those initial case reports with the FDAs Office of Compliance in the Center for Devices and Radiological Health, continued to provide updates as more details became available, provided a full briefing of its investigation to the FDA on April 5 and filed a Medical Device Report on April 7.
Given the scientific and epidemiological data, B&L concludes that the formulation of MoistureLoc itself may increase the risk of developing Fusarium, hence the decision to discontinue the product.
Another possible scenario: Improper contact lens care, including failure to clean and replace lenses regularly, use the solution properly and replace the cases regularly. Call it the perfect storm, says optometrist Jack Schaeffer, incoming chairman of the AOAs Contact Lens and Cornea Section.
Meanwhile, the FDA and CDC both say that there are no problems with B&Ls ReNu MultiPlus or ReNu Multi-Purpose solutions or with generic brands. The company further says that it is currently working with the FDA to take corrective actions in B&Ls manufacturing plant based on the FDA inspection.
The FDA says it will continue to review cultures and epidemiological data. Meanwhile, at least 19 lawsuits, mostly class-action suits, have been filed against B&L, reports the Rochester Democrat and Chronicle, of Rochester, N.Y.
One possible bright spot: With all the publicity, patients are being made aware of the importance of proper care, Dr. Schaeffer says.
The AOA has a section about Fusarium, including a review of the infection and patient handouts, on its Web site at www.aoa.org/x5119.xml.

