 |
|
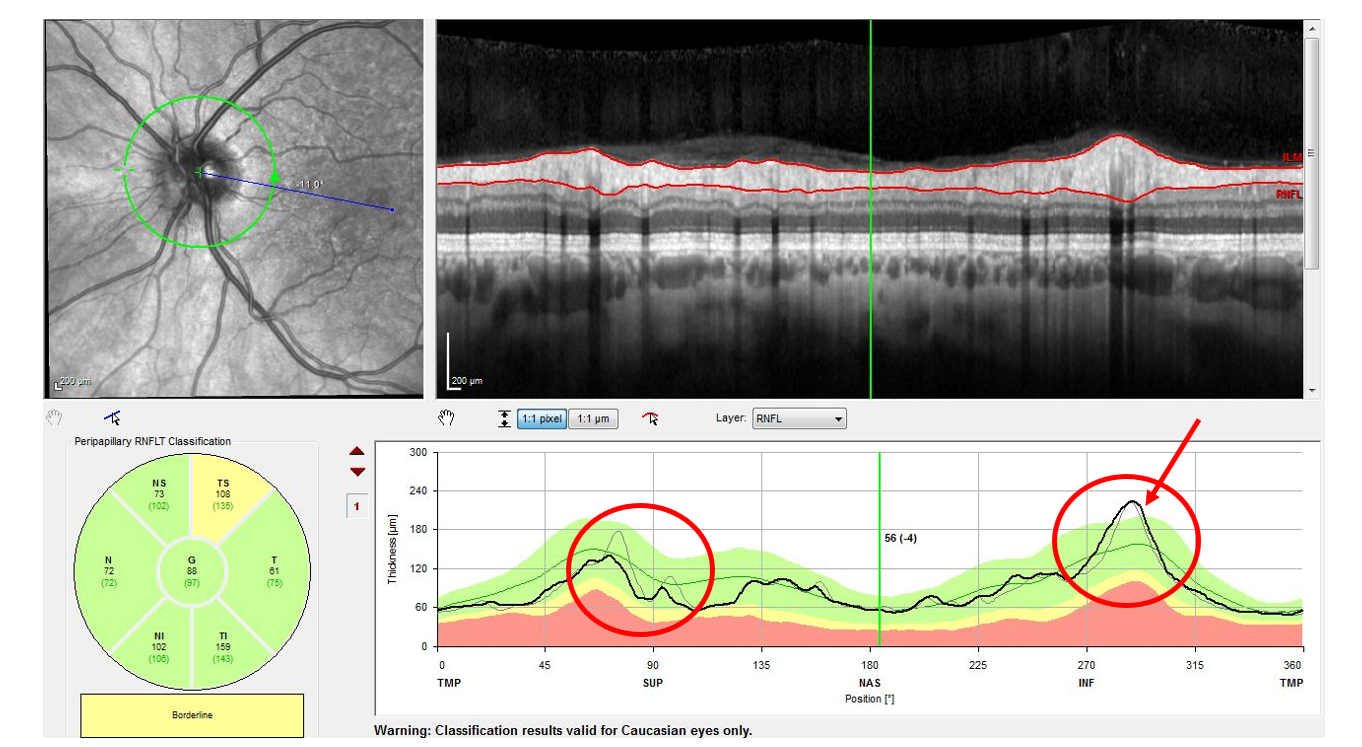
Ocular biomarkers may serve as ideal for testing remyelination efficacy of drugs, as in this MS patient who demonstrates evidence of optic neuritis on OCT. Photo: Wang B, et al. J ASCO, 44(3), Summer 2019. Click image to enlarge. |
Highly effective immunomodulatory disease-modifying therapies have greatly helped treat those with multiple sclerosis (MS). However, there is still a need to combat ongoing disability progression outside of periods of relapse. This brings to the forefront the possibility of remyelination through certain agents with the hope of preventing neurodegeneration and promoting repair.
Consequently, researchers wanted to investigate through meta-analysis how these newer remyelinating agents are being measured and used in clinical trials. A recent Journal of Neuro-ophthalmology literature review revealed that there is rapid expansion in studies testing remyelinating candidates, but further growth is limited due to lack of consensus on trial designs. There is currently no defined ideal study population, therapy duration or appropriate outcome measures to detect remyelination. As such, the study authors argue that biomarkers of remyelination need to be developed and validated to serve as surrogate endpoints in clinical trials.
This is where ocular involvement comes in. Due to visual system dysfunction being common in MS and contributing to overall disability, the eye serves as a great window to study remyelination agent efficacy. Dynamic injury and recovery along the visual pathway are reflective of the brain, and both acute and chronic demyelination of the visual system can be studied. Therefore, the authors propose certain ocular biomarkers may serve as proxies for overall remyelination with these agents.
The first is neuroimaging. While most conventional MRI sequences lack the sensitivity necessary to detect myelin content changes, MRI imaging of optic nerve lesions may complement existing measures of optic nerve remyelination.
The next category involves visual outcomes, which includes visual evoked potentials (VEPs), OCT and functional visual outcomes. VEPs have recently demonstrated that latency corresponds with measures of nodal structure and extent of myelinated axons on both inflammatory and chemical models of demyelination. Also found in mice was that VEP latency improved after clemastine treatment, supporting latency as a preclinical biomarker for validation of putative remyelinating agents.
Using OCT, the ganglion cell layer, inner plexiform layer and peripapillary retinal nerve fiber layer have been studied well in MS. While thinning of these layers is thought to reflect axonal injury and neurodegeneration, they do not directly detect myelin changes. Instead, these OCT measurements may capture a downstream neuroprotective benefit or other off-target effect.
Low-contrast letter visual acuity (LCLA) can detect meaningful vision changes in those with MS and has been found in studies to improve with immunomodulatory therapies. However, this raises concern that the method may capture off-target anti-inflammatory effects instead of reflect remyelination. LCLA improvements can support the clinical relevance of putative remyelinating agents, but other electrophysiologic data or biomarkers with greater pathologic specificity will be needed.
Finally, the option of detecting visuomotor abnormalities may be a possibility. One study used a custom-built retinal eye tracker to quantify fixational microsaccade features which could accurately determine MS patients from controls; microsaccade number indicated measures of disability.
The authors relay that “key advantages of using visual assessments in remyelination trials include their low cost, sensitivity and accessibility. Well-established functional, electrophysiologic and structural measures allow for multimodal assessment of the visual pathway.”
They recognize that, “although these measures may have varying specificity for remyelination, a combination of techniques applied in either acute or chronic demyelination may increase our understanding of the interplay between inflammation, remyelination and neurodegeneration in MS.”
Zuroff LR, Green AJ. The study of remyelinating therapies in multiple sclerosis: visual outcomes as a window into repair. J Neuroophthalmol. April 24, 2024. [Epub ahead of print]. |


