| |
Volume 17, Number 1 |
March 2021 |
|
FROM
THE DESK OF THE EDITOR
Here we are rolling into the spring of 2021! Vaccine administrations are steadily increasing and with that comes a renewed sense of optimism. My home in central Indiana has just seen its first few days of spring weather. Most of the country was hit with a brutal snowstorm and frigid cold weather in late February. To see the weather here now hit the mid-60s has been a pleasant turn of events. The Indiana kids have already switched their wardrobe to shorts and T-shirts. It is amazing what sunshine and vitamin D does for the body and our mental health. As COVID fatigue has set in for so many people, vaccines and spring weather are the perfect combination to get our spirits back up. March marks the one-year anniversary of COVID turning our worlds upside down. This is when our states went into emergency action and our clinics had to close down to routine care. Even though that anniversary is depressing to consider, I am optimistic. I’m not sure if that was a side effect of my second dose of the Moderna vaccine or if I’ve just been vitamin D deprived for too long? But either way, there seems to be hope on the horizon.
In this March edition of the ORS newsletter, I’d like to draw your attention to a few items. First, we have a new bunch of officers, introduced below, that will be serving the ORS over the next two-year cycle. Congratulations to them all! Secondly, I want to point out the what’s your diagnosis case. Now I may be biased as the case report was written by my current resident. But I think the case is quite unique and has not yet been described in the retinal literature. This was a great case for our resident to come across. It may have been a challenging and unique year for residents and students, but I’m so glad to see that they are still getting the same quality clinical education.
Cheers!
Anna Bedwell, OD, FAAO, FORS
Editor-in-Chief
PRESIDENT'S MESSAGE
It is my pleasure to serve as the new President of the Optometric Retina Society (ORS). First, I wanted to thank the outgoing officers Dr. Jeff Austin and Dr. Julie Torbit for their past service and continued dedication and support of ORS. I also would like to welcome our new officers: Dr. Christopher Suhr, VP; Dr. James Williamson, secretary; Dr. Julie Rodman, treasurer, as well as the remaining and new ORS Committee members. We are all committed to continue carrying the mission of the ORS.
Despite the challenges of 2020, with continued affiliation with the Review Education Group, we conducted ORS’s annual Retina Update meeting in the virtual space successfully, with over 200 attendees. A number of our courses are being offered as enduring web-based CE for several more months.
Going forward one of our goals is to raise awareness about ORS among our optometric colleagues, students and residents, and to boost our membership. I encourage all of you to inspire peers that are, or whom we can help to become, eligible to obtain ORS fellowship, and help them realize the prestige of joining this esteemed group and society. We need broad recognition of the designation F.O.R.S. in the rank of other familiar optometric organizations. I am confident we all can help to achieve this goal.
With wishes of good health, security, and prosperity for all of you.
Mohammad Rafieetary, OD, FAAO, FORS
President, Optometric Retina Society
ORS New Officer Introduction (serving through December 2022)
|
|
Dr. Mohammad Rafieetary
President |
|
Dr. Christopher Suhr
Vice President |
|
|
|
Dr. James Williamson
Secretary |
|
Dr. Julie Rodman
Treasurer |
|

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

Image Gallery
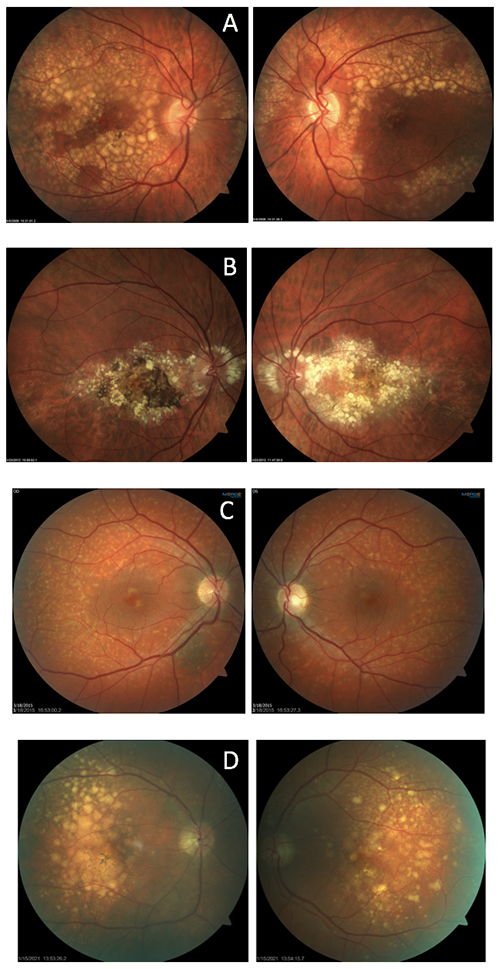
Which of the following set of images is NOT an example of Doyne’s honeycomb dystrophy (aka Malatia Leventinese)?
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Sex Differences in Presentation, Treatment Patterns, and Clinical Outcomes in Central Retinal Vein Occlusion
Central retinal vein occlusion (CRVO) is a commonly encountered ocular condition that can cause retinal ischemia and/or macular edema. This retrospective study specifically analyzed cases of CRVO that had occurred over a seven-year period to look for differences between males and females. The study reviewed 476 CRVO cases with a roughly equal split between males and females (47% vs. 53%, respectively). The study found males at risk to develop CRVO at a younger age than females (63.8 years vs. 66.1, p=.048) and at greater risk for ischemia. The central subfield thickness on OCT was statistically thicker in males at both baseline and final visits. Notably, current smokers held a higher risk of CRVO in the males over the female patients. Otherwise, the risk factors were similar between the two groups. Both groups experienced similar visual outcomes and held comparable risk for CRVO in the fellow eye.
This study showed that there are differences in clinical characteristics of CRVO between males and females while outcome tends to be similar across patient sex. Of clinical importance, tobacco use was a significant risk factor for CRVO in males. As tobacco use is a modifiable risk factor, this emphasizes the importance of cessation counseling to our patients.
Mirzania D, Thomas AS, Rothman AL, et al. sex differences in presentation, treatment patterns, and clinical outcomes in central retinal vein occlusion. Ophthalmic Surg Lasers Imaging Retina. 2020; May 1;51(5):279-85.
The Incidence of Neovascularization in Central Serous Chorioretinopathy by Optical Coherence Tomography Angiography
Central serous chorioretinopathy (CSC) runs a risk of leading to development of neovascularization, particularly in chronic cases. Detecting neovascularization (NV) early in CSC with traditional fluorescein angiography has limitations. This retrospective review looked at the incidence of NV detected via optical coherence tomography angiography (OCTA), a tool which has better sensitivity to uncover NV in CSC cases. Of the 175 eyes that met the inclusion, there were 86 acute and 89 chronic cases, defined by symptoms greater than six months with OCT features of chronicity. Eyes were divided into two groups: those without NV (n=140) and those with NV (n=35).
All of the 35 eyes that developed NV had chronic CSC, amounting to 39.2% incidence of NV in chronic CSC and 20% incidence in total CSC cases. The NV was classified as type 1 in all the cases. Of those, seven eyes (20%) were silent, nonexudative NV indicating that OCTA was a critical tool to detect the NV. Older age and greater choroidal thickness had a stronger association with the development of NV.
Detecting NV from CSC can be challenging with traditional fluorescein angiography, as dye leakage from CSC could hide NV visualization. The authors found a 20% incidence of NV in CSC, higher than previous reports. This may be attributable to superior NV detection in CSC cases via OCTA, stressing the clinical utility of OCTA in monitoring chronic CSC for early NV detection.
Savastano MC, Rispoli M, Lumbroso B. the incidence of neovascularization in central serous chorioretinopathy by optical coherence tomography angiography. Retina. 2021; Feb 1;41(2):302-8.
Retinal Vascular Manifestations of Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) is reported to affect 22% of men and 17% of women worldwide, and approximately 75% of individuals with a BMI greater than 40 kg/m2. A large number of people with OSA are undiagnosed, and the vast majority of individuals with the disease are untreated. In addition to having a substantial negative impact on cardiovascular diseases such as stroke, myocardial infarction, atrial fibrillation, hypertension, and diabetes, OSA can also impact retinal vascular disease. It is believed to do so as a result of hypoxia, oxidative stress, and activation of the sympathetic system. In particular, OSA can increase the likelihood of or worsen diabetic retinopathy, retinal vein occlusion, and central serous chorioretinopathy. In patients with diabetic retinopathy, OSA can hasten the progression of non-proliferative retinopathy to proliferative disease and lead to an increase in the chance of developing diabetic macular edema. Retinal vein occlusion is also more likely to occur in those with OSA, with the occlusion most frequently occurring overnight and the vision loss being noticed upon awakening. This is believed to be due to a combination of increased hypercoagulability and increased blood pressure. When a patient is newly diagnosed with a retinal vein occlusion, OSA should be strongly considered as a potential underlying factor. Finally, OSA also increases the likelihood of developing central serous chorioretinopathy, most likely as a result of increased sympathetic activation and increased oxidative stress. Thankfully, effective treatment of the OSA via a CPAP device often leads to rapid improvement in the serous retinopathy.
D'Souza H, Kapoor KG. Retinal vascular manifestations of obstructive sleep apnea. Curr Opin Ophthalmol. 2020; Nov;31(6):508-13.
Retinal Microvascular Abnormalities in Patients after COVID-19 Depending on Disease Severity
SARS COVID-2 has the potential to affect multiple organs. This study was undertaken to evaluate its effect on the retina. Patients in the study were between 18 and 55 years old, and had PCR-confirmed SARS-COVID 2 infection within three months of enrollment (except for the normal controls). Exclusion criteria were previously known ophthalmological findings, systemic diseases known to be associated with retinopathy, and myopia greater than -6.00 diopters. The COVID patients were divided into three groups. Group 1) mild disease with minimal or no symptoms, group 2) moderate disease with hospitalization but no acute respiratory distress, and group 3) severe disease with ICU admission for acute respiratory distress. The study included 96 total patients. There were 27 control patients who had not had COVID, 24 patients in group 1, 24 patients in group 2, and 21 patients in group 3. All study participants had a non-mydriatic color photo, OCT, and OCTA performed. There were no visible retinal abnormalities seen on any patient via photography, other than a single retinal hemorrhage seen in one patient who was taking heparin. All OCTs were normal other than two patients with small, asymptomatic pigment epithelial detachments. On OCTA testing, there was decreased central vessel density of the superficial vascular plexus in patients with moderate and severe disease, which was not seen in patients with mild disease or in the normal control subjects. Though the sample size is small, these findings seem to suggest that moderate to severe COVID infection can affect the retinal vasculature.
Zapata MÁ, Banderas García S, Sánchez-Moltalvá A, et al. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol. 2020; Dec 16. [Epub ahead of print].
Increasing Incidence of Macular Edema in Excessive Morning Blood Pressure Surge in Patients with Retinal Vein Occlusion
The goal of this study was to understand the way blood pressure impacts the potential for macular edema in patients with retinal vein occlusion (RVO). RVO occurs when a thrombosis forms at an arteriovenous crossing or at the optic disc, leading to a blockage of the venous system. Blood pressure, a known association with RVO, varies during each 24-hour period with blood pressure decreasing during sleep and increasing in the morning. In this study, 76 patients diagnosed with RVO underwent hospital treatment, 24-hour ambulatory blood pressure monitoring (ABPM), carotid intima-media thickness testing (CIMT), and pulse wave velocity testing (PWV). Extreme morning blood pressure surge (MBPS) was associated with macular edema. The cutoffs for MBPS were 29.3 mmHg for sleep-trough MBPS and 14.4 mmHg for pre-waking MBPS. Sleep-trough MBPS was measured as the average morning systolic blood pressure (SBP) of the first two hours after waking, subtracted by the average of the lowest nocturnal SBP. Pre-waking MBPS was the average of the morning SBP of the first two hours after waking, subtracted by the average of the SBP taken the two hours prior to waking up.
This prospective study had limitations, as it was small and included patients from only one practice. However, it did show that certain aspects of blood pressure monitoring may be a useful predictor of macular edema in patients with RVO, specifically the MBPS. It also found that MBPS was independent of blood pressure as a predictor for macular edema. This is important because even patients with blood pressure considered to be normal may be at higher risk for edema based on BP fluctuation. The authors acknowledged that more work needs to be done to assess what this means for treatment and management, but it’s an important step to understand which patients are at risk and how their physiology impacts the management and treatment of RVO.
Kim HJ, Shin YU, Lee Y, et al. Increasing incidence of macular edema in excessive morning blood pressure surge in patients with retinal vein occlusion. Sci Rep. 2020;10(1):4420.
The Impact of Race on Short-term Treatment Response to Bevacizumab in Diabetic Macular Edema
This was a retrospective study that pointed to the inequities seen in eye care and the healthcare field in general, and the importance of using data to inform our decision making going forward. This study was conducted in an urban teaching facility in Boston, an institution that has a diverse patient base. This study compared the results of bevacizumab injection in black, white and Hispanic patients that have diabetic macular edema. The inclusion criteria were 18 years of age or older, first-time anti-VEGF treatment, diagnosis of macular edema, no laser treatment within three months of the study and no other invasive treatments used to treat diabetic retinopathy. Data was collected at baseline, one to three months following the initial injection, and three months after the third injection; 314 charts were collected for single injection analysis and 151 charts were used for three-injection analysis.
The study found that fewer black patients (26.71%) had an improvement in visual acuity (VA) of at least 0.1 on the logMAR scale than white (50%) or Hispanic (39.39%) patients after the initial injection. After the third injection, the trend remained the same. Only 33.82% of black patients experienced an improvement in VA, while 57.76% of Hispanic patients and 58.84% of white patients saw an improvement in VA. The study pointed out that this data is not altogether surprising considering that anti-VEGF treatments were created for and tested on patients with age-related macular degeneration, a disease that disproportionally affects white patients. The interesting aspect of the data was that while the improvement in VA was statistically significant among the groups, the decrease in central macular thickness (CMT) was not. In other words, all groups had a decrease in macular thickness that was similar. The decrease in CMT was 12.30% for black patients, 17.01% for Hispanic patients, and 20.66% in white patients after the first injection. The decrease in CMT after the third injection was 24.36% for black patients, 16.13% for Hispanic patients and 25.38% for white patients. Studies show that black patients have an increased risk of developing clinically significant macular edema yet they have significantly poorer response to the most popular and most affordable anti-VEGF drug on the market. This study was conducted from 2010 to 2019, and it is reportedly the first study to look at the impact of race on anti-VEGF treatment. This study reminds us that further research needs to be done on anti-VEGF treatment so each patient can get optimal and effective care.
Osathanugrah P, Sanjiv N, Siegel NH, et al. The Impact of race on short-term treatment response to bevacizumab in diabetic macular edema. Am J Ophthalmol. 2021; Feb;222:310-17.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
Balversa-induced Retinopathy
Introduction
Balversa (erdafitinib) is an oral chemotherapy agent that was approved for use in the US in 2019. It is a kinase inhibitor that is used to treat urothelial carcinoma. Although there are no absolute contraindications, two major side effects include ocular disorders and hyperphosphatemia. This case study will explore the possible ocular side effects of Balversa and the plausible mechanisms of action by which these occur.
Case Report
A 78-year-old white male, presented to the clinic for the first time. The patient had no specific chief complaint; he was referred by his oncologist. He had an ocular history of a constant right 45 prism diopter exotropia and cataract extraction in both eyes. The patient had high blood pressure, type 2 diabetes, chronic kidney disease, and urothelial carcinoma.
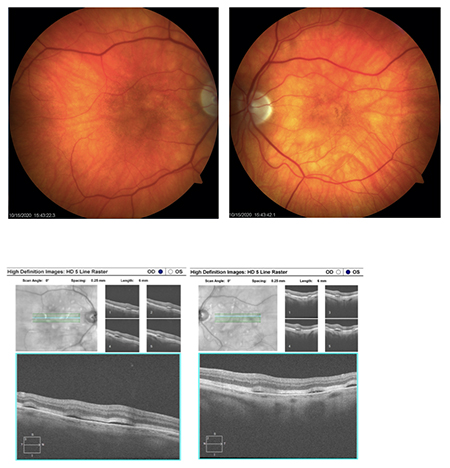
Visual acuity was 20/50 OD and 20/30-1 OS. Intraocular pressure was 22 OU with Goldmann applanation tonometry. Anterior segment was unremarkable. Posterior examination revealed uneven pigmentation and elevation throughout the macular and posterior pole. Multiple pigment epithelial detachments were seen throughout the posterior pole. Fundus photos and SD-OCT were taken (see images at top of newsletter).
Differentials
Multiple PEDs due to Balversa, idiopathic multiple PED syndrome, age-related macular degeneration (AMD), central serous chorioretinopathy (CSR), polypoidal choroidal vasculopathy, hypertensive choroidopathy, choroidal tumors.
Discussion:
Ultimately, the patient was diagnosed with multiple PEDs related to Balversa use. This patient started taking Balversa as a treatment for urothelial carcinoma one month prior to his appointment. Based on FDA reports, CSR/PED as a result of Balversa use occurs in 25% of patients.1 Ocular complications can occur quickly, with an average onset of 50 days after starting Balversa.2 The mechanism of action behind the formation of CSR or PED during Balversa use is unclear. It is plausible that the drug causes increased cortisol levels, which have been linked to development of PED and CSR.3 Additionally, as a kinase inhibitor, Balversa may interfere with choroidal or retinal vasculature or RPE integrity.4 The FDA released a scale that can be used to grade the severity of the two major ocular complications caused by Balversa. Grade 1: asymptomatic, clinical diagnosis; observation only. Grade 2: visual acuity of 20/40 or better, or ≤3 lines of decreased vision from baseline. Grade 3: visual acuity worse than 20/40, or >3 lines of vision decrease from baseline. Grade 4: visual acuity of 20/200 or worse.1 Grading for this patient was confounded by the presence of a pre-existing constant exotropia and unknown baseline visual acuities. It may be prudent for patients to have a required eye exam shortly before starting Balversa. A baseline exam would allow practitioners to better use the grading scale established by the FDA after treatment initiation.
Treatment
According to FDA guidelines, patients taking Balversa should have a dilated exam once a month for the first four months of treatment, and every three months thereafter. If CSR or PED are discovered, the FDA recommends the patient discontinue Balversa until the PEDs resolve. If a patient is categorized as Grade 1 to 3, the medication may be reinstated once PED or CSR have resolved. If a patient has deterioration of vison to 20/200 or worse, they are considered Grade 4, and the medication should be discontinued permanently.1 The examination findings were sent to the patient’s oncologist, and after discussion with the oncologist’s office, the patient was taken off Balversa. The patient was meant to return for a dilated exam one month from the initial encounter, but was hospitalized for other medical issues and subsequently passed away.
Conclusion
PED and CSR are relatively common side effects of Balversa, a medication used in the treatment of urothelial carcinoma. It is important that clinicians who see patients taking Balversa are aware of theses potentially vision-threating side effects and understand the best course of treatment. Ultimately, more research needs to be done to better understand Balversa-induced retinopathy.
Meret Thomas-Huebner, OD
Resident,
Indianapolis Eye Care Center
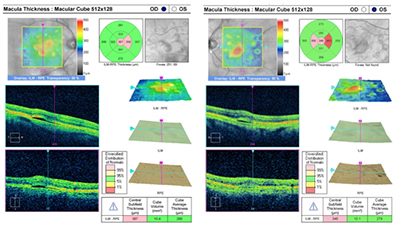 |
| Image (above): OCT macular thickness map of each eye highlighting the multiple serous PEDS OD and OS. |
References:
1. Highlights of Prescribing Information. FDA.
2. Drug Trails Snapshots: BALVERSA. FDA.
3.Goncu, T, Ozdek, S. Idiopathic multiple tiny serous retinal pigment epithelial detachments: Report of 3 cases and review of the literature. Optometry. 2011;82:556-62.
4. Kransnicki, P, et al. Seven-year evaluation of idiopathic multiple retinal pigment epithelium detachments. GMS Ophthalmology Cases. 2016;6:12. |

IN THE
NEWS
|
 Is In-Home OCT Monitoring on the Horizon? Is In-Home OCT Monitoring on the Horizon?
Remote OCT monitoring has many potential benefits to optimize treatment outcomes while decreasing patient office visits, a burden for patients and busy doctors’ offices alike. Notal Vision, Inc. has been on the forefront of this push since their home-based OCT system was granted FDA Breakthrough Device designation in late 2018. Notal recently announced the start of the first US study of their home-based system in 15 participants with wet AMD. The study seeks to assess participant’s abilities’ to navigate and operate the device in home for daily self-imaging. All of the subjects will be under retinal specialist care with anti-VEGF therapy for wet AMD and tracked over a 90-day span, while study investigators monitor the cases remotely. Genentech (part of Roche Group) is partially funding the study.
|
|
 AsclepiX Partners with Notal Vision for In-home OCT Monitoring in Clinical Trials AsclepiX Partners with Notal Vision for In-home OCT Monitoring in Clinical Trials
If patients can self-image retinal OCTs at home, then can that be harnessed for research monitoring as well? Notal Vision and AsclepiX Therapeutics announced in February a partnership between the two companies to apply in-home OCT into AsclepiX’s Phase I trial of AXT107. Notal Vision will provide the Home OCT monitoring service during the trial of subjects treated with AXT107 for either DME or AMD.
|
|
 Adverum Projects Shorter Timeline for Gene Therapy in Wet AMD Adverum Projects Shorter Timeline for Gene Therapy in Wet AMD
Adverum announced its alignment with the FDA in clinical development of their intravitreal gene therapy candidate, ADVM-022, for wet AMD. The data from the OPTIC trial has been favorable, showing an 85% reduction in anti-VEGF injections. Adverum now plans to start two Phase III clinical trials (900 total patients) to study different doses of ADVM-022 compared to aflibercept every eight weeks. Adverum anticipates a Biologics License Application submission to the FDA in 2024. |
|
 Iridex to Collaborate with Topcon Iridex to Collaborate with Topcon
Iridex, a developer of ophthalmologic lasers and instrumentation, announced its alliance with Topcon. In the new relationship, Topcon will invest in Iridex and in turn purchase rights to distribute Iridex’s retina and glaucoma products across certain worldwide markets. Iridex will also acquire Topcon’s PASCAL® product line with the intention to combine the technologies between Iridex’s MicroPulse® and Topcon’s PASCAL® laser platform.
|
|
 Faricimab Shows Promising Phase III Data Faricimab Shows Promising Phase III Data
Genentech announced positive results in their four Phase III studies on faricimab, an agent that targets angiopoietin-2 (Ang-2) and VEGF-A. These studies include the TENAYA and LUCERNE studies in wet AMD, and the YOSEMITE and RHINE studies in DME. About half of those eligible for extended dosing on faricimab were able to extend injections to every four months over the first year. Study results were virtually presented at Angiogenesis, Exudation, and Degeneration 2021.
|

|
IMAGE QUIZ ANSWER
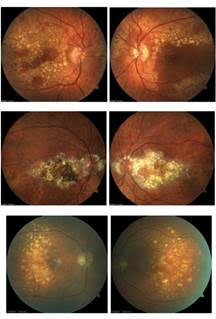 |
This is a twist on the typical image quiz serving as an opportunity to showcase the unique clinical appearance of Doyne’s dystrophy. The correct answer, letter C, shows a case of fundus flavimaculatus. The remaining three sets of images (on the left) are patients with Doyne’s dystrophy, also known as Malattia Leventinese.
The dystrophy is characterized by onset of drusen in young to mid-adulthood. The drusen develop into a honeycomb type pattern localizing to the macula and peripapillary. As the drusen develop and coalesce, the eyes generally look symmetric, as demonstrated by these images. Doyne’s is caused by an autosomal dominant mutation in the EFEMP1 gene. Often, Doyne’s is misdiagnosed as age-related macular degeneration because of the drusen. However, the two diagnoses can be differentiated based on age of onset and genetic testing for a mutation in the EFEMP1 gene. Genetic testing has become more readily available for those with inherited retinal dystrophies and serves as an important tool for proper genetic counseling.
Similar to AMD, there is risk for choroidal neovascularization (CNV) and geographic atrophy in patients with Doyne’s. There is no treatment for Doyne’s, unless CNV develops.
A: Doyne's B: Doyne's C: fundus flavimaculatus D: Doyne's
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Meret Thomas-Huebner, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|