| |
Volume 15, Number 2 |
June 2019 |
|
|
Inside
This Issue |
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
I will keep this message short, as typing one-handed holding a newborn baby is somewhat challenging! We have all noticed, whether fully aware or not, the rapidly increasing use of artifical intelligence or AI. By definition AI means the intelligence generated by a machine to replicate human thinking. Essentially, a machine that can learn and problem solve in a manner similar to you and I. As I am currently spending time at home on a maternity leave, I have honed in on the AI all around me (particularly as the internet constantly bombards me with advertisments for diapers and baby clothes). On the flipside, AI allowed me the exhilerating opportunity to ride in a Tesla on Autopilot. Now AI is steadily moving into healthcare, including eyecare. Have a read below in the journal abstract section to get yourself up to speed on AI and its ophthamological applications.
Finally, I’d like to send appreciation to my colleagues, Brad Sutton and Jim Williamson, as well as my resident, Daniel Bollier, who help me put this newsletter together. I appreciate all their assistance to get the newsletter out on time while I am home on maternity leave. I’d truly be lost without all their help.
Anna Bedwell, OD, FAAO
Editor-in-Chief
PRESIDENT'S MESSAGE
I was recently discussing photography with a friend of mine who is just beginning to enter the hobby. As we discussed the nuances of aperture size and ISO, my part of our conversation evolved quickly to how amazing the eye is as a camera system. We discussed how it automatically adjusts aperture size to optimize the light entering the eye and then on to the amazing retina and the “adjustable ISO” (or film speed for the pre-digital photographers among us) and then the retinal circuits that are accomplished by the horizontal connections in the retina to give the ability to fine tune the spatial sensitivity with negative surround. I was really stretching to remember some of my physiological optics knowledge from optometry school, but I think I got some of it right. I made the point that one would think that this type of visual tuning would be accomplished in the higher visual centers of the brain, but most of it is done right in the retina which is considered a fairly low neurologic level. I repeated to him the retinologists’ cliché that the rest of the eye really is a support system for the retina, and the retina is about the only major part of the eye that, for the most part, we cannot repair once damaged. He said that his mother had macular degeneration. I then told him to make sure to eat his green leafy vegetables, take the right supplements, not smoke and wear his sunglasses to help keep his retinas healthy.
As optometrists, we are blessed to be able to help people maintain the gift of sight by keeping their wonderful retinas (and the rest of the support system) healthy and functioning properly. Hopefully one day we will have the ability to repair all retinal damage, but until then, we’ll all just have to do the best we can to help our patients understand the best ways to protect their valuable sight. The gift of sight truly is amazing.
Jeffrey Austin, OD, FAAO
ORS President

Book Release
OCT Angiography is the one of the newest tools in ocular disease management. OCTA allows for non-invasive detection of choroidal neovascularization, early diabetic retinopathy and much more.
“Optical Coherence Tomography Angiography Atlas: A Case Study Approach,” by ORS fellow, Dr. Julie Rodman, offers an excellent guide to to the technology with illustrated case examples to show its clinical applications. This is the only book on this topic written by an optometrist. The book is available for purchase on Amazon, Barnes and Noble and through SLACK publishers.
|
YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.
Answer appears later in newsletter.

Image Gallery
Which of the following images represents bear tracks?
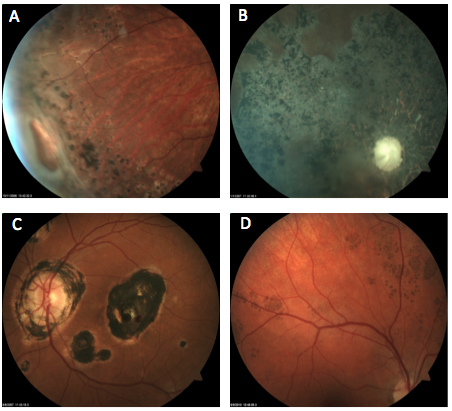
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Detection of Clinically Unsuspected Retinal Neovascularization with Wide-field Optical Coherence Tomography Angiography
Current optical coherence tomography angiography (OCTA) systems have a limited scan area, either 3x3 mm or 6x6 mm. Thus, they are not as helpful in detecting neovascularization (NV) in proliferative diabetic retinopathy, as NV often falls outside the scan area. This small, prospective clinical trial looked at the utility of using wide-field OCTA compared to conventional OCTA for detection of NV in diabetic retinopathy.
All study participants had a pre-existing diagnosis of nonproliferative DR (13 mild, 7 moderate and 7 severe). A masked retinal specialist determined the severity of NPDR based off 7-field ETDRS color photos. Two separate, masked graders evaluated the WF-OCTA (five 6x10mm scans, montaged to 25x10mm) and conventional OCTA (three 6x6mm scans, montaged to 15x6mm). The wide-field OCTA found evidence of retinal NV in four eyes (15%). The traditional OCTA found retinal NV in two eyes (7%), but only with the 6x6mm central scan and not the 3x3 mm scan. Of those four eyes with NV, two were clinically graded as moderate, but by 7-field color photos, all four were graded as severe.
This study showed that OCTA was capable of detecting subtle retinal NV that was not appreciable in color photos or on physical exam. WF-OCTA had the advantage over conventional OCTA of detecting retinal NV, which is not surprising as it provides a greater field of view. Interestingly, three of the four eyes (vs. 3/23 eyes without NV) with retinal NV on OCTA had history of anti-VEGF injection for diabetic macular edema over two months prior to the current study. This suggests that routine screening with WF-OCTA for subtle NV may be of use particularly in severe NPDR or in those undergoing anti-VEGF injection for DME.
You QS, Guo Y, Wang J, et al. Detection of clinically unsuspected retinal neovascularization with widefield optical coherence tomography angiography. Retina. 2019; Mar 4. doi: 10.1097/IAE.0000000000002487. [Epub ahead of print].
Earliest Evidence of Preclinical Diabetic Retinopathy Revealed Using OCT Angiography (OCTA) Perfused Capillary Density
Optical coherence angiography (OCTA) allows for a three-dimensional analysis of the retinal microvascular. Could it potentially act as a tool to screen for preclinical diabetic retinal vascular changes? Researchers looked to answer this question by specifically assessing parafoveal perfused capillary density (PCD) as a potential marker for preclinical diabetic retinopathy.
This study involved 40 normal eyes without pathology and without major vascular disease compared to 112 eyes in patients with diabetes. The diabetic eyes either had no clinical evidence of retinopathy (36), NPDR (38) or PDR (38). No eyes had active macular edema. In each participant, a 3x3mm OCTA image was obtained and then processed by manual delineation of the foveal avascular zone (FAZ) and automatic segmentation to remove noncapillary blood vessels. This created a PCD map that was then sectioned off into seven annuli rings for data comparison.
In analyzing PCD, there were significant differences between each group, most specifically in the central 200-micron annulus. PCD was greatest in the no retinopathy group, then control, NPDR and PDR groups. When analyzing FAZ, the PDR group had a significantly greater FAZ. There was no statistical difference in FAZ metrics between the control and no retinopathy groups.
This study looked at capillary PCD, as opposed to other studies that have looked at PCD values with capillary and noncapillary vessels. Interestingly, researchers found increased PCD values in diabetic eyes without retinopathy over the control eyes, which might signify a response to increased metabolic demand. This was followed by a decline in PCD as loss of capillaries occurred in NPDR and PDR. The shift from rise to drop of PCD might mark the shifting point for development of clinical evidence of disease. This suggested that PCD values from OCTA might be of importance in detecting preclinical diabetic retinopathy.
Rosen RB, Andrade Romo JS, Krawitz BD, et al. Earliest evidence of preclinical diabetic retinopathy revealed using oct angiography (octa) perfused capillary density. Am J Ophthalmol. 2019; Jan 25. doi: 10.1016/j.ajo.2019.01.012. [Epub ahead of print].
Early Hydroxychloroquine Retinopathy: Optical Coherence Tomography Abnormalities Preceding Humphrey Visual Field Defects
In the majority of cases with documented retinal Plaquenil toxicity, both structural (OCT, etc.) and functional (VF) changes can be identified. There have been several reports, however, of visual field defects preceding OCT loss. This case series is the first to report the opposite: eyes in which OCT damage appeared before any visual field loss.
The retrospective review included charts from seven tertiary retina centers and identified 17 eyes of 10 patents that, at some point, exhibited OCT changes but no visual field loss. All of the patients were at increased risk for retinopathy due to total cumulative dose, excessive daily dose above 5mg/kg/day of actual body weight, or long duration of use.
The early OCT defects were either attenuation of the parafoveal ellipsoid zone (photoreceptor integrity line), loss of an identifiable parafoveal interdigitation zone, or both. None of the involved eyes exhibited visible fundus changes or abnormal fundus autofluoresence (FAF), but several did show an abnormal multifocal ERG. Over time, many of the 17 eyes went on to develop the classic “flying saucer” sign on OCT and/or paracentral VF loss, thus further verifying that the initial OCT changes seen prior to VF loss did indeed represent early Plaquenil damage. So while this report included only a small number of patients, it appears that some individuals taking Plaquenil can exhibit OCT damage prior to any identifiable visual field loss.
Garrity ST, Jung JY, Pichi F, et al. Early hydroxychloroquine retinopathy: optical coherence tomography abnormalities preceding Humphrey visual field defects. Br J Ophthalmol. 2019 Feb 28. doi: 10.1136/bjophthalmol-2018-313350. [Epub ahead of print].
Optical Coherence Tomography Angiography Analysis of Macular Flow Density in Glaucoma
As OCTA technology has evolved, new clinical uses continue to emerge. It has been well-established that glaucoma is a multifaceted disease process, with ocular blood flow being one component. OCTA is uniquely suited for the noninvasive study of ocular blood flow parameters. This study from Germany compared the macular blood flow density of 30 patients with glaucoma to 21 normal, matched controls using Heidelberg Spectralis OCTA and custom analysis software.
Both the superficial retinal vascular plexus and the deep retinal vascular plexus were analyzed. The macular blood flow density was found to be significantly reduced in glaucoma patients compared to normal patients, with the difference being the most pronounced in the nasal sector where the papillomacular bundle is located. The size of the foveal avascular zone was also compared between the two patient groups, but no significant difference was found. The investigators further compared macular blood flow density to the location of visual field defects, RNFL thickness and overall retinal thickness. No significant correlations were found in any of these areas.
Though the sample size was small and custom analysis software was required, this study indicates the future potential to include macular blood flow density parameters in the clinical management of glaucoma.
Kromer R, Glusa P, Framme C, et al. Optical coherence tomography angiography analysis of macular flow density in glaucoma. Acta Ophthalmol. 2019 Mar;97(2):e199-e206.
Rapid Progression of Cataract to Mature Stage After Intravitreal Dexamethasone Injection: a Case Report
This case report involved a 59-year-old Korean man who suffered a branch retinal vein occlusion in his left eye, with extensive macular edema and BCVA of 20/200. Three days after the onset of decreased vision, he received scatter laser photocoagulation and an intravitreal dexamethasone implant (Ozurdex). One month later, the macular edema had improved, and best corrected vision was 20/40. Unfortunately, three months after the implant, the edema and vision worsened, and a second Ozurdex implant was administered. The same pattern repeated itself, with initial improvement followed by worsening, so a third Ozurdex implant was given approximately four months after the second (around eight months after the occlusion).
Prior to the third intravitreal steroid implant, the lens was clear with no signs of cataract. One week after the third implant, a grade 1 PSC had formed and IOP had elevated to 42mmHg. Treatment with oral acetazolamide and topical drops lowered the IOP to 18, but two weeks after that, the cataract had progressed to mature status with no view through the lens and hand motion vision.
The authors noted this to be the first report of very rapid formation of a dense cataract after intravitreal steroid implantation, with a clear lens turning in to a mature cataract in three weeks. However, it should be remembered that this was the third intravitreal steroid implant given to this individual, and several months had passed after the first and second ones were injected. So while it is true that the cataract formed very rapidly after the third steroid implant, the pump had certainly been primed, so to speak, with the prior two implants, likely impacting the timing of the cataract’s formation.
Lee JH, Park JY, Kim JS, et al. Rapid progression of cataract to mature stage after intravitreal dexamethasone injection: a case report. BMC Ophthalmology. 2019; Jan 3;19(1):1.
Choriocapillaris Hypoperfusion Artifact in OCT Angiography
Optical coherence tomography (OCT) angiography (OCTA) is a noninvasive way to assess pathology in the retina. It uses OCT technology to assess the movement of red blood cells in the lumen of the vessels throughout the retina and choroid. There are great advantages to this technology, but also many disadvantages. OCTA is noninvasive, fast, and depth-resolved. Fluorescein angiography and indocyanine green angiography only provide two-dimensional images, but also detect leakage, unlike OCTA. OCTA can identify and help diagnose many forms of retinal conditions.
This paper described the potential of misinterpreting OCTA if not carefully assessing the OCTA alongside a still image to potentially identify artifacts. A corresponding structural image needs to be evaluated side by side with the en face flow image if there is a reduction in the flow signal. This can help eliminate any potential misinterpretations.
Six eyes of six different patients in this study had different disorders of the macula. All patients showed decreased flow signal in the choriocapilaris (CC). The signal loss was due to different disorders in the macula such as macular serous detachment, hyperreflective lesions in the middle retinal layers, drusen, thickened and hyperreflective RPE, and subretinal hyperreflective material. When the structural image was compared with the corresponding en face flow image there was a decreased flow of the CC beneath the pathology in the retina. It was not possible to confirm the presence of the change of flow because the signal in the same region of the still image was also diminished. The conclusion of a decrease in perfusion in the CC cannot be made since there was a correlating decrease in the structural image. It was found that the greater the hyperreflectivity of the inner retina, the more it affected the signal in the CC.
Authors wrote that, while manufacturers are making improvements and adjustments to help reduce the artifacts and errors of OCTA, it is important to use the structural image when analyzing a suspicious area of decreased flow on the angiogram. They added that this would help avoid falsely diagnosing flow alterations in the CC.
Kayat KV, Roisman L, Zett C at al. Choriocapillaris hypoperfusion artifact in oct angiography. ophthalmic surg lasers imaging retina. 2018 Aug 1;49(8):603-610.
Paracentral Acute Middle Maculopathy and the Ischemic Cascade Associated with Retinal Vascular Occlusion
OCT and OCTA allow for depth segmentation capability, a feature fluorescein angiography lacks. This offers better understanding of the intermediate (ICP) and deep retinal capillary (DCP) plexi, the vascular region affected in paracentral acute middle maculopathy (PAMM). By OCT definition, PAMM is a hyperreflective band involving the inner nuclear layer (INL) caused by ICP/DCP ischemia. The purpose of this retrospective observational case series was to identify the pattern of ischemia in patients with PAMM from retinal vascular occlusion via analysis of en face OCT images.
Patients in this study had some form of large-vessel retinal vascular occlusion. Sixteen eyes of 16 patients were evaluated. En face OCT imaging was used to segment the INL. The authors described two types of PAMM pattern: a perivenular fern-like distribution and a diffuse globular distribution. Patients with the perivenular fern-like PAMM had the best resultant visual acuity when compared with globular pattern of PAMM. Neither PAMM pattern correlated with the type of retinal vascular occlusion. There were two cases observed where there was pattern progression from initial perivenular fern-like PAMM to diffuse globular correlating with a progressive decline in visual acuity.
The authors observed a continuum in this study that was found to start in the deep capillary plexus in the inner nuclear layer (INL), with perivenular fern-like PAMM moving to involve the entire INL (globular PAMM) and finally spreading to the inner retina in the superficial capillary plexus. The most susceptible area to damage and lack of oxygen was found in the middle retina in the deep capillary plexus. The visual outcome reflected this cascade as well.
Bakhoum MF, Freund KB, Dolz-Marco R et al. Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 2018 Nov;195:143-153.
Artificial Intelligence in Retina
Given diabetic retinopathy (DR) is recognized as a worldwide epidemic and age-related macular degeneration (AMD) affects 170 million people worldwide, these authors took a comprehensive look into artificial intelligence (AI) in retina. The termed AI was coined in 1956 at the so-called Dartmouth workshop, which is considered its birthplace. Later in 1959, machine learning (ML) emerged by developing algorithms that could extract generalized principles from data.
A recurring theme in ML research is imitation of the neural structure of the central nervous system (CNS). The deep learning (DL) architecture found most suitable for imaging data is the convolutional neural networks (CNNs). The authors state that trained with large annotated datasets, CNNs essentially allowed computers to start recognizing visual patterns and are primarily responsible for the recent resurgence and overwhelming interest in AI.
A significant boost in the ability of computers to recognize image content came through the ImageNet Large Scale Visual Recognition Competition. In this event, the goal was to automatically classify more than 1.2 million natural images and photographs into 1,000 categories. By 2015, DL CNN models developed were reported to reach the human level of ability at such a specific “task” of image identification.
The authors note that the retina expert needs to become aware of the large spectrum of ML and DL methods, which will be the basis of clinical management decisions. Previous studies such as the ETDRS and AREDS were based on human feature discrimination. Hence, AI represents a major paradigm shift in retinal diagnosis.
Other medical fields have already highlighted the benefits of AI in their environments: CNN could detect tuberculosis in chest radiographs, melanoma from skin photographs more accurately than dermatologists and metastatic cells in lymph node samples more precisely than pathologists.
The authors say data access and, therefore, big data sharing are quintessential issues in ML and DL. The other “elephant in the room” is the black-box phenomenon. In DL, it is challenging to understand how exactly a neural network reaches a particular decision, or to identify which exact features it utilizes. The black-box phenomena is particularly intrinsic to daily routine as digital imaging focuses on subclinical biomarkers such as hyperreflective foci, deep capillary plexus and other features beyond clinically visible correlates. Hence, AI-based detection and integration is not an alternative but a necessity, the authors state.
Schmidt-Erfurth U, Sadeghipour A, Gerendas B, et al. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1-29.
Introduction to Machine Learning for Ophthalmologists
The authors explain the commonly used terms in machine learning (ML) along with different types of variables and classifiers. They discuss data science as the process of collecting data, extracting relevant information, presenting new insights and taking automated data-based decisions. They explain that this term acts as an umbrella that includes artificial intelligence (AI), machine learning (ML), and neural networks. The authors review the above in detail using charts and analogies.
Support Vector Machine (SVM) has recently become one of the most popular supervised learning classifiers due to its good outcomes when classifying difficult examples. The authors state that, in its most general form, SVM is a linear classifier, meaning that it seeks to find a hyperplane (e.g., a line for a dataset with two variables, a plane for a dataset with three variables, etc.) that perfectly separates data points belonging to different classes. Although there are many possible hyperplanes, the classifier selects the one that has the biggest margin that separates it from data points, the authors wrote.
The authors reviewed ML for glaucoma, diabetes, DR and AMD, which has been studied by others. In addition, they mentioned several other diseases for ML. For dry eye, they stated that diagnostic methods are usually slit lamp- or questionnaire-based. ML techniques have shown their potential to standardize dry eye diagnostics. Classification of 55 infrared meibomian gland images by means of image processing and linear SVM achieved specificity of 96% and sensitivity of 98% when differentiating between dry eye and healthy eyes, the authors say.
Cataracts, authors wrote, are often assessed by comparing a slit lamp exam to a set of standardized photographs (e.g., LOCS III). A method to grade cataracts based on SVM was proposed by several researchers. When applied to a set of 5,750 slit-lamp images, the performance of the automatic method was more successful (99.4% images correctly graded) than the subjective method (97.0%).
The authors concluded that the large number of imaging and image processing techniques available nowadays presents new opportunities to develop decision-support tools that assist clinicians with the diagnosis of almost any ocular condition.
Consejo A, Melcer T, Rozema J. Introduction to machine learning for ophthalmologists. Semin Ophthalmol. 2019;34(1)19-41..
Automated Detection of Macular Diseases by Optical Coherence Tomography and Artificial Intelligence Machine Learning of Optical Coherence Tomography Images
Optical coherence tomography (OCT) is essential for retinal evaluation. Its development provided a breakthrough in the ability to diagnose macular diseases, and assess the necessity and efficacy of treatments.
Artificial intelligence (AI) has been applied to engineering platforms such as facial recognition systems, self-driving cars and automated voice guidance. Machine learning (ML) is a computational technology programmed to recognize patterns from a large data set. Deep learning (DL) is one of the machine learning techniques, with a multilayered convolutional neural networks (CNN) model to learn and detect image features. In this study, the authors evaluated the feasibility of a CNN model trained on the basis of DL with image augmentation by horizontal flipping, rotation and translation for automated detection of macular diseases from OCT images.
A total of 1,200 OCT images from 600 eyes were retrospectively collected from a single hospital. A single reader provided one diagnosis to a pair of OCT images in a masked fashion. When there were multiple diagnostic suggestions, the most pathological diagnosis was recorded. The diagnoses involved normal eyes (n=570) and those with wet age-related macular degeneration (AMD) (n=136), diabetic retinopathy (DR) (n=104), epiretinal membranes (ERMs) (n=90) and another 19 diseases. Among them, 1,100 images were used for DL training, augmented to 59,400 by horizontal flipping, rotation and translation. The remaining 100 images were used to evaluate the trained CNN model. The authors used Caffe, an open-source CNN framework, for DL.
The CNN model identified a variety of macular diseases involving vitreomacular traction syndrome, ERM, BRVO, DR, CSC, wet AMD, VKH disease and posterior staphyloma. A CNN model is likely to recognize both structural changes (vitreous traction, macular holes, serous retinal detachments and RPE detachments) and exudative changes (hyperreflective foci and subretinal hemorrhages).
One of the major issues of this study is the small number of images for training. A characteristic feature could be learned even with a small number of images sufficiently to facilitate disease identification, such as VKH disease and posterior staphyloma. On the other hand, variations of minimal pathologic findings might need a larger number of samples for training.
Soichiro K, Ayatsuka Y, Yanagisono et al. Automated detection of macular diseases by optical coherence tomography and artificial machine learning of optical coherence tomography images. Open Access J Ophthalmol. 2019;2019:1-7. https://doi.org/10.1155/2019/6319581
Automated Diabetic Retinopathy Detection in Smartphone-based Fundus Photography Using Artificial Intelligence
Individuals with diabetes require regular monitoring for early detection of diabetic retinopathy (DR) in order to optimize visual outcomes. Usually this occurs with a dilated exam from an eyecare professional or through nonmydriatic photographs taken as a screening exam. However, smartphone-based retinal imaging has emerged as one of the recent cost-effective ways of screening for retinopathy in the community.
Using artificial intelligence (AI), the detection and grading of DR occurs after the machine exposes itself to thousands of retinal images. The authors note that to their knowledge, assessment of the use of AI along with smartphone-based fundus photography has not been done so far.
A total of 301 patients with type 2 diabetes, ages 18 years and above, undergoing treatment for diabetes at a tertiary care diabetes hospital with varying duration of diabetes underwent fundus photography using the Remidio Fundus on Phone (FOP), a smartphone-based imaging device (Remidio Innovative Solutions Pvt. Ltd, Bangalore, India). The FOP, which provides a 45-degree field of view, can be used in handheld mode or attached to a slit lamp. Four fields were captured in each eye on the FOP camera: macula centered, disc centered, superior-temporal and inferior-temporal. Automated analysis occurred with the EyeArt software (EyeNuk). EyeArt is a cloud-based software program that can automatically analyze retinal images, and provide DR severity and screening recommendation by automatically detecting the presence, size, position and number of retinal lesions.
The authors reported a high sensitivity for detection of DR, sight-threatening DR (STDR) and referable DR (RDR) (above 95% for DR, 99% for STDR and RDR) using the EyeArt software when used on retinal images taken with FOP. This is similar to the high sensitivity in the Google AI algorithm, which showed a high sensitivity and specificity for RDR when used on conventional retinal photography. The authors noted a lower specificity for RDR due to a higher estimation of moderate NDPR from non-DR, false-positive lesions such a drusen, RPE hypertrophy, retinal vein occlusion and RPE atrophy.
The authors concluded that, in this study, despite no formal machine learning/training of the algorithm on the images from FOP, the EyeArt solution was able to grade images for STDR with a high sensitivity of 99.1% and specificity of 80.4%. They suggested that the false-positive cases could then be excluded on referral.
Rajalakshmi R, Subashini R, Anjana R, et al. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye. 2018;32:1138-1144.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
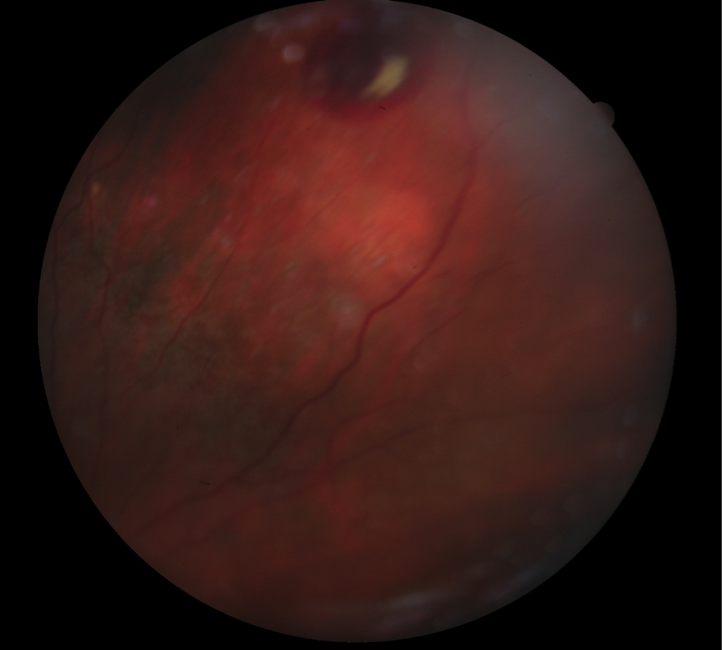
A 79-year-old-white female presented to clinic with complaints of difficulty reading at close distances and seeing faces at far distances in both eyes. She presented to our geriatric clinic with the following findings: visual acuities were 20/80 OD and OS. Pupils were equal, round and reactive to light, with no APD. Confrontation fields were full to finger count OD, OS, and her EOMS were full range of motion OU. Slit lamp examination was remarkable for PCIOLs OU. DFE of the right eye posterior pole revealed macular geographic atrophy and a blot hemorrhage 1.5 DD superior to the optic nerve. DFE of the left eye posterior pole revealed macular geographic atrophy, and few dot-blot hemorrhages inferior to the optic nerve. Mid-peripheral retinal evaluation of both eyes revealed various areas of RPE alterations, which resembled intra-retinal hemorrhages. Peripheral retinal evaluation of the right eye revealed a subretinal hemorrhage and scar due to a resolved subretinal hemorrhage. Peripheral retina evaluation of the left eye revealed a scar due to a resolved subretinal hemorrhage. The patient was referred for evaluation to a retinal specialist, who diagnosed peripheral exudative hemorrhagic chorioretinopathy (PEHCR) and dry AMD OU. The retinal specialist’s plan was to follow the patient without treatment.
Top differential diagnoses for PEHCR are retinal capillary hemangioma, retinal macroaneurysm, retinal telangiectasia, choroidal detachment and choroidal melanoma, with choroidal melanoma being the most common misdiagnosis for PEHCR. In a study evaluating 12,000 patients, PEHCR was identified in 13% of patients referred with a diagnosis of uveal melanoma.1,2 In Mantel et al.’s series, 86.6% of 56 eyes presented with an initial diagnosis of choroidal malignancy. Because choroidal masses are the most common misdiagnosis for PEHCR, Carol Shields highlighted clinical features that can be used to distinguish between PEHCR and choroidal masses, including retinal exudation, macular and peripheral RPE alterations, absence of sentinel vessels on anterior segment, appearance of hypofluorescence on FA and absence of intrinsic vascular pulsation on ultrasonography.1
PEHCR is a rare degenerative disease of the peripheral retina3, and is predominantly seen in elderly women and accompanied by hemorrhage and/or exudation.4 Advancing age serves as a risk factor for development of PEHCR. Fluctuating systemic blood pressure and use of anticoagulants are additional risk factors for hemorrhages and play a role in recurrent hemorrhages.5 The clinical features of PEHCR can be described based on peripheral and macular pathology. The peripheral features of PEHCR include peripheral subretinal and/or subRPE hemorrhages, exudation, retinal pigment epithelial detachment and lesions located in the temporal quadrant. The macular pathological features of PEHCR include macular drusen, RPE alterations, choroidal neovascularization and decline in visual acuity if the lesions progress from the periphery to the macula. PEHCR lesions are commonly located in the temporal retina between the equator and the ora serrata.1,3,6 Visual acuity in PEHCR can be affected due to presence of intravitreal hemorrhages, macular edema and/or accompanying AMD-related findings such as drusen, CNV and geographic atrophy. Shields et al. observed age-related macular changes in 17% of same eyes and 27% of contralateral eyes in PEHCR patients.1 PEHCR is diagnosed by patient history, ophthalmoscopic findings and testing. Fundus fluorescein angiography findings include hypofluorescence consistent with hemorrhage in lesion area and irregular hyperfluorescence shown by CNV and homogenous hyperfluorescence typical of serous PED in the periphery. Indocyanine green angiography can show polypoid formations and abnormal choroidal vasculature.3
Usually, PEHCR is stable or spontaneously regresses, which can result in atrophy, fibrosis or hyperplasia.1 The treatment options for PEHCR include: monitoring, photocoagulation, photodynamic therapy, cryotherapy, intravitreal anti-VEGF and vitrectomy.7,8
The prognosis of PEHCR depends on the location of the lesion. The disease may spontaneously regress in cases where the central vision or macula remain unaffected, so following without intervention is recommended.9 Laser photocoagulation or PDT is reserved for cases that are threatening the macula or peripheral polyps.10 A pars plana vitrectomy is mandated in rare cases of vitreous hemorrhage or massive subretinal hemorrhage.9
Marlon Demeritt, OD, MBA, FAAO
ORS fellow
 |
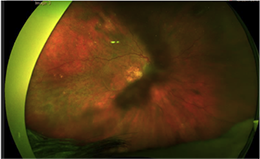 |
Figure 1. (Earlier in newsletter.) Fundus photograph of OD revealed peripheral subretinal hemorrhage.
Figure 2. Fundus photograph of OD revealed geographic atrophy and midperipheral RPE alterations.
Figure 3. Fundus photograph of OD revealed geographic atrophy, midperipheral RPE alterations, scattered drusen and isolated peripheral subretinal hemorrhage.
1. Shields CL, Salazar PF, Mashayekhi A, et al. Peripheral exudative hemorrhagic chorioretinopathy simulating choroidal melanoma in 173 eyes. Ophthalmology. 2009;116:529-35.
2. Shields JA, Mashayekhi A, Ra S, Shields CL. Pseudomonas of the posterior uveal tract: the 2006 Taylor R. Smith. Retina. 2005;25:767-71.
3. Mantel I, Schalenbourg A, Zografos L. Periperhal exudative hemorrhagic chorioretinopathy: polypoidal choroidal vasculopathy and hemodynamic modification. Am J Ophthalmol. 2012;153:910-22.
4. Annesley WH. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-64.
5. Cebec Z, Dere Y, Bayraktar S, et al. Clinical features and course of patients with peripheral exudative hemorrhagic chorioretinopathy. Turk J. Ophthalmol. 2016;46:215-20.
6. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical, angiographic, and histologic study. Am J Ophthalmol. 2009;148:932-8.
7. Alforja MS, Sabater N, Giralt J, et al. Intravitreal bevacizumab injection for peripheral exudative hemorrhagic chorioretinopathy. Jpn J Ophthalmol. 2011;55:425-7.
8. Pinarci EY, Kihe I, Bayer SA, et al. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27:111-2.
9. Reese AB, Jones IS. Hematomas under the retinal pigment epithelium. Am J Ophthalmol. 1962;53:897-910.
10. Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008;19:208-12.
|

IN THE
NEWS
 |
FDA Approves Eylea for All Stages of Diabetic Retinopathy
According to a press release from Regeneron, the FDA has approved aflibercept for all stages of diabetic retinopathy (DR). As such, Eylea is the only vascular endothelial growth factor (VEGF) inhibitor approved with two dosing options for DR, allowing doctors to customize treatment to their patients' needs. In DR, Eylea may be dosed every eight weeks following five initial monthly injections, or every four weeks. The FDA approval of Eylea as a treatment for DR was based on six-month and one-year results from PANORAMA, a randomized, multi-center, controlled Phase III trial that enrolled 402 patients and was designed to investigate Eylea for the improvement of moderately severe to severe NPDR without diabetic macular edema (DME), compared to sham injection. PANORAMA is the first prospective trial to study whether an anti-VEGF can also help prevent worsening disease in patients with NPDR without DME.
|
 |
Results in from the Treatment for Center-Involved (CI) Diabetic Macular Edema (DME) in Eyes with Very Good VA Study (Protocol V)
Finally, some evidence-based guidelines for patients with CI-DME and relatively unaffected visual acuity. According to the Protocol V study results, patients with CI-DME and good vision can be managed by observation with anti-VEGF injections occurring only if vision decreases. The 702 participants presented with visual acuity of 20/25 or better and were placed into groups of observation, laser or Eylea injections. At two years, the rates of visual acuity loss of five or more letters did not differ significantly between groups. Patients in the laser and observation groups were monitored closely at weeks eight and 16, and then at 16-week intervals. They were switched to Eylea if a decrease of two or more lines at any visit, or one line at two consecutive visits was reported.
|
 |
EyePoint Launches New Treatment Option
EyePoint Pharmaceuticals released Yutiq, a new treatment option for chronic non-infectious posterior uveitis. The intravitreal implant is a non-bioerodible micro-insert that contains 0.18 mg fluocinolone acetonide, designed to release 0.25 mcg/day over the course of 36 months. Yutiq can be administered in a physician’s office through a single-dose preloaded applicator. The company says that peaks and valleys associated with typical treatment options are avoided, as the implant consistently releases the steroid for three years.
|
 |
PubMed Approves Ophthalmology Retina for Indexing
The National Library of Medicine has accepted Ophthalmology Retina for inclusion in Medline/PubMed, the first time it has accepted a printed, monthly U.S. ophthalmology journal in 12 years. Launched two years ago, the American Academy of Ophthalmology created Ophthalmology Retina in response to the growing volume of high-quality research within the retina subspecialty of ophthalmology. Ophthalmology Retina is the first new Academy journal since the Transactions of the American Academy of Ophthalmology and Otolaryngology was initially published in 1903. The journal publisher, Elsevier, is working with Medline/PubMed to have Ophthalmology Retina articles appear in Medline in about four to six weeks.
|
 |
World Health Organization (WHO) Adds Burnout to ICD-11
Emphasizing that burnout is an occupational phenomenon and not a medical condition, the World Health Organization (WHO) has included burnout in the International Classification of Diseases, 11th Revision (ICD-11). According to WHO, burnout can be characterized by three dimensions: Feelings of energy depletion or exhaustion; increased mental distance from one’s job, or feelings of negativism or cynicism related to one’s job; and reduced professional efficacy. While burnout was also included in ICD-10 in the same category, the definition is now more detailed.
|
| |
| |
|

|
IMAGE QUIZ ANSWER
Bear tracks, answer D, are characterized by small, multiple congenital hypertrophy of the retinal pigment epithelium (CHRPE) lesions clustered together. The appearance resembles paw or track prints, hence the name. The lesions are darkly pigmented and flat.
Bear tracks are considered benign but must be differentiated from the RPE lesions associated with familial adenomatous polyposis and Gardner’s syndrome, which closely resemble CHRPE.
A: laser retinopexy
B: syphilitic chorioretinopathy
C: presumed ocular histoplasmosis (POHS)
|
MEET THE FELLOWS
Marlon J. Demeritt, OD, MBA, FAAO, received an associate in arts degree from Miami-Dade Community College in 1998, and completed his requirements for a bachelor of science degree with concentration in biological sciences in 2000 from FIU. He graduated from Nova Southeastern University College of Optometry in 2004 with a doctor of optometry degree. After graduating, he completed a one-year residency at The Malcom Randall VA & UF Shands, in affiliation with the NSU College of Optometry.
After completion of his residency, Dr. Demeritt worked for several practices. Dr. Demeritt gained extensive and invaluable experience as a consultant for patients pursuing refractive surgery. In addition, Dr. Demeritt worked in subspecialty ophthalmology practices conducting pre- and post-exams for cataract surgery patients, managing glaucoma and retina patients, and serving as the director of the dry eye service at East Florida Eye Institute located in Stuart, FL. In addition to serving as the Director of The Dry Eye Service, Dr. Demeritt also served as a sub-investigator for glaucoma trials, Inotek: Adenosine Inhibitor.
In August 2012, Dr. Demeritt joined the NSU Faculty as adjunct professor precepting third- and fourth-year students. In February 2013, Dr. Demeritt joined the Nova Southeastern faculty on a full-time basis as an assistant professor.
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
SPONSOR NEWS

Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Daniel Bollier, OD
Contributor
Jim Williamson, OD, FAAO
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|