| |
Volume 16, Number 4 |
December 2020 |
|
FROM
THE DESK OF THE EDITOR
The holiday season has hit. But it’s just not the same this year. Not all has been merry and bright as we’ve battled a pandemic over the past nine months that has turned all our lives upside down. Many of us will have some time off at the end of the year. I, for one, look forward to spending time with my immediate family and taking a mental break from coronavirus (is that even possible?). As I remind myself that I have a lot to be thankful for: my family, our health and finally getting OCTA in our office. I hope that many of you all are able to do the same.
This year has been rough, but it has reinforced some positive messages. For one, I never thought of optometry as an “essential” career. I knew my job was important, but I guess essential never really crossed my mind, until 2020. Eyecare is essential, because our vision is essential. Whether you’re in clinical practice, research, teaching or industry, we are all working hard to provide access to top-notch eyecare. Just look at the news briefs section below; it is an amazing reminder of the exciting treatments and technology on the horizon for retinal care. Coronavirus may have slowed us down, but we’re all just as committed as ever to finding the best care for our patients.
Happy Holidays! Cheers to 2021!
Anna Bedwell, OD, FAAO, FORS
Editor-in-Chief
PRESIDENT'S MESSAGE
The year of the Eye Doctor, 2020, is coming to a close...thank goodness! We all hope for a much better 2021. I try to be an optimist, and look for the good in life and in people. As I ponder the difficulties that we have all endured over the past nine months of this pandemic, I am ever grateful that I live today in a time where we have the technology to produce a vaccine in amazing record time to help stem the tide of this horrible infection. That vaccine will likely begin to be distributed and administered as you read this newsletter. Never before in the history of humankind has a vaccine been developed in such a short time. One life lost is too many, but I am so grateful that we will in short order be able to prevent much sickness and many more deaths.
We just concluded the annual ORS Retina Update continuing education meeting this past weekend. We had a wonderful slate of speakers and very informative lectures. Many of these lectures are online at the Review of Optometry CE website for credit if you missed the meeting. Again, as I ponder the technology that we have available to us today, I am overwhelmed with gratitude for living in these times. OCT was unheard of when I graduated optometry school. How much have OCT and other technological advances changed and improved eye care over the past 10 to 20 years? We can diagnose things more accurately and much earlier. Treatments and outcomes are better than they have ever been. This gives me great hope for even better diagnostic and treatment options in the future. Just as I am optimistic about eye care, so am I optimistic that we will have a much better year in 2021. I wish all of you a happy, safe and healthy holiday season and new year.
Jeffrey Austin, OD, FAAO, FORS
President, Optometric Retina Society

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

Image Gallery
Which of the following images represents paracentral acute middle maculopathy (PAMM)?
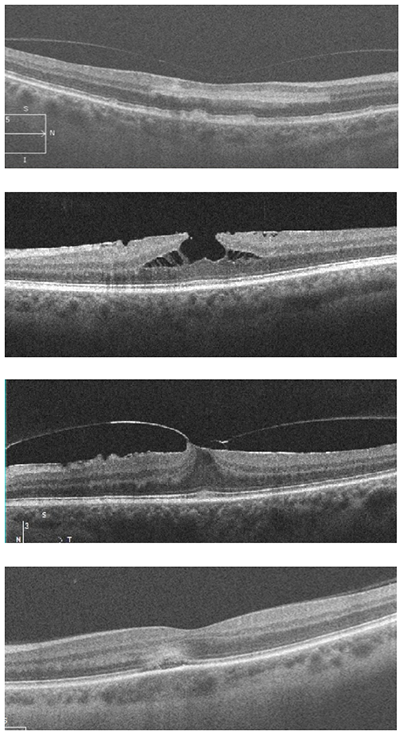
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Five-Year Outcomes After Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study)
While clinical trials consistently show benefit in anti-VEGF treatment of center involved diabetic macular edema (CI-DME), some argue that the gains are not as substantial in the real-world experience. Protocol T followed patients over two years of treatment, with one eye randomized to either aflibercept, bevacizumab, or ranibizumab and showed favorable results. But what happens to these patients after the trial ends?
This report detailed the clinical outcomes for Protocol T patients at five years after randomization. After two years of study participation, the patients were returned to treatment based on clinician discretion. Of those eligible, 68% (317/463) fulfilled the five-year visit. In the latter three-year period, the median number of injections was four. Some patients maintained the same treatment as during the study, while others were switched to another anti-VEGF agent or not treated. The mean five-year VA improved for all participants in comparison to baseline (7.4 letters). However, there was a decline of about one line of acuity (4.7 letters) between year two to five. Though VA was still better at five years compared to baseline. Approximately half of patients were 20/25 or better at five years and only 5% were 20/200 or worse. There was no significant difference in mean retinal thickness between two and five years.
This study showed that patients at five years had better VA compared with baseline. However, the VA found at two years was not maintained once Protocol T ended and patients switched to clinician directed treatment. More insight is necessary to determine how best to maintain visual acuity gains for patients with CI-DME without the need for structured protocol of frequent injections.
Glassman AR, Wells JA 3rd, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020; Sep;127(9):1201-10.
Comparing Intravitreal Air and Gas for the Treatment of Vitreomacular Traction
Posterior vitreous detachment (PVD) is an inevitable process in the aging population. During PVD the macula is susceptible to complications such as vitreomacular traction (VMT), epiretinal membrane or the worsening of pre-existing macular disease. VMT has been of particular interest. Though it may spontaneously resolve over time, there is also the risk of bothersome symptoms or progression to macular hole formation. Injection of an air or gas bubble has been prosed as potential treatment to consider over vitrectomy.
This was an interventional, nonrandomized, consecutive case series that compared air and gas (C3F8) in the treatment of symptomatic VMT. Excluded were patients with a history of retinal break, a refraction greater than -6D or axial length greater than 25 mm. Treatment consisted of intravitreal injection of either 0.3 mL C3F8 or 0.3 mL filtered air. Twenty-two eyes were included with a total of 24 injections (two eyes received two injections). At one month follow up, VMT released in 11 patients. Of those, seven of 11 (64%) C3F8 injections and three of 13 (23%) air injections. There was a statistically significant decrease in central retinal thickness in the VMT release group compared to the non-release group. There was a negligible improvement in visual acuity in those with successful release. Of note, one case (C3F8 group) had elevated IOP post-treatment, which was successfully managed. Another case, in the non-release group, progressed to MH formation.
This study provides more encouraging data to support treatment of symptomatic VMT with intravitreal injection of gas. The results here show superiority to air, which may relate to the density of gas and ability to manipulate the vitreous cortex to induce PVD. Other small studies have demonstrated similar successful release rates with C3F8 varying from 40 to 96% success. More study is necessary to determine which symptomatic VMT cases are more likely to have success. Intravitreal gas injection may offer a superior alternative to Jetrea to consider before pursuing surgical intervention with vitrectomy.
Gruchociak S, Djerada Z, Afriat M, et al. Comparing Intravitreal air and gas for the treatment of vitreomacular traction. Retina. 2020;40(11):2140-7.
Update in Soft-Tissue Filler-Associated Blindness
Ocular vascular occlusion and subsequent blindness are very rare but devastating complications of cosmetic soft-tissue filler injection. Such fillers include, but are not limited to, hyaluronic acid, autologous fat and calcium hydroxyapatite. When injected on the face or forehead, inadvertent intra-arterial injection can result in particles making their way into the ocular vasculature via retrograde circulation.
This article reviewed English language literature reports on this topic published between January 2015 and August 2018. For reference, in 2017, an estimated 1.6 million total filler injections were performed. During the 2015-2018 time period reviewed, there were 60 unique cases of soft-tissue-filler-associated vision loss reported. Interestingly, 16.7% of those cases experienced simultaneous ischemic neurological stroke. During this time period, the most commonly injected filler was hyaluronic acid. Ocular involvement included cases of central retinal artery occlusion, choroidal emboli, ophthalmic artery occlusion, branch retinal artery occlusion and posterior ischemic optic neuropathy. Ophthalmic artery occlusion was the most common. Ophthalmoplegia and skin necrosis also occurred. Of the 60 cases reported with ocular vascular occlusion, only 10 reported vision restoration after treatment. The most common and most effective treatment was injection of hyaluronidase when the filler used was hyaluronic acid. Injection of hyaluronidase was the most effective when administered very shortly after ocular symptoms began, often within just a few minutes. A delay of even a few hours resulted in much worse results. Other attempted treatment modalities included hyperbaric oxygen, ocular massage, acetazolamide and systemic antibiotics. While soft-tissue-filler-injection-induced ocular complications are very rare, it is imperative for practitioners to be aware of this potentially devastating side effect and act quickly in an attempt to restore vision.
Sorensen EP, Council ML. Update in soft-tissue filler-associated blindness. Dermatol Surg. 2020 May;46(5):671-7.
Using the Thickness Map from Macular Ganglion Cell Analysis to Differentiate Retinal Vein Occlusion from Glaucoma
Acute retinal vein occlusion and glaucoma are very easily distinguished from one another. When a hemicentral or branch retinal vein occlusion occurred in the past, however, it can be very difficult to ascertain that fact when vision is normal and no collateral vessels developed. In that instance, residual visual field loss from the vein occlusion can be mistaken for glaucomatous visual field defects. The authors of this article postulate that ganglion cell analysis utilizing macular OCT scans may be able to distinguish old, non-apparent vein occlusions from glaucoma. The retrospective study evaluated 37 patients with resolved retinal vein occlusion affecting one hemifield and 74 patients with primary open angle glaucoma. They theorized that ganglion cell defects related to glaucoma would be smooth and arcuate, while those from retinal vein occlusion would be irregular and less arcuate. Two masked examiners were asked to evaluate OCT ganglion cell thickness maps and classify the patient as either having POAG or having had a remote retinal vein occlusion. They were not provided with any other information or findings, and made their determination based only upon the OCT ganglion cell findings. To be included, patients had to have BCVA of 20/30 or better and no history of ocular pathology other than POAG or retinal vein occlusion. The examiners were able to differentiate retinal vein occlusion from POAG with a specificity of 93.2% and a diagnostic accuracy of 80.4%. The authors postulated that OCT ganglion cell thickness maps are an inexpensive and accurate tool for distinguishing POAG from old retinal vein occlusion. This has important clinical implications, as visual field defects due to vein occlusions are stable and non-progressive, thus requiring no intervention.
Lee NH, Park KS, Lee HM, et al. Using the thickness map from macular ganglion cell analysis to differentiate retinal vein occlusion from glaucoma. J Clin Med. 2020;9(10):3294.
Blood Pressure Variability in Patients with Branch Retinal Vein Occlusion
Retinal vein occlusion (RVO) is pervasive and can significantly compromise vision, yet the pathogenesis is still unknown. There have been numerous risk factors reported, and one of the mostly widely known is hypertension. However, normotensive patients can also suffer from RVO. This study sought to better understand the role blood pressure plays in development of RVO. There is evidence that suggests fluctuation, blood pressure variability (BPV), throughout the day is an independent risk factor for RVO. BPV can be separated into two different categories: long-term BPV and short-term BPV. Short-term BPV is the change in BP from minute to minute, and from day to night over a 24-hour period. Long-term BPV is change in BP over several days to several years.
This study focused on short-term BPV in two groups: patients with BRVO (80 patients) and the control group (75 patients). The participants in each group were matched in sex and age. Patients with other posterior segment disease were excluded as were patients taking any other systemic medication aside from anti-hypertensives. The ambulatory blood pressure of members in each group was measured for 24 hours. A BP measurement was taken every 15 minutes during the day and every 30 minutes at night. The mean daytime, nighttime and 24-hour values for systolic, diastolic and average blood pressure were not significantly different between groups. But the mean variability index was significantly greater among the BRVO group. Other studies have shown that high BPV is a predictor of early progression of atherosclerosis. BPV also likely causes ocular hemodynamic disruption leading to problems with ocular perfusion pressure. This study demonstrated a significant difference between the variability in blood pressure during a 24-hour period between a group of patients with BRVO and a control group. The group with BRVO had a significantly higher BPV compared with the controls. The authors suggested monitoring BP for a longer period of time may provide information about how BPV affects the prognosis of patients with BRVO.
Gulmez M, Tekce A. Blood pressure variability in patients with branch retinal vein occlusion. Retina. 2020 Oct;40(10):2045-9.
The Clinical Features and Genetic Spectrum of a Large Cohort of Chinese Patients with Vitelliform Macular Dystrophies
This study explored the phenotypic and genotypic variations of vitelliform macular dystrophies in a group of 134 Chinese patients. There are three commonly identified vitelliform dystrophies: best vitelliform (BVMD), autosomal recessive bestrophinopathy (ARB) and adult vitelliform (AVMD). Each of these dystrophies is linked to mutations of the BEST1 gene. Each patient was diagnosed via dilated exam and EOG. Information such as age of onset and family history was also reported. Patients diagnosed with BVMD had juvenile to adult onset, macular vitelliform lesions and abnormal EOG with an Arden ratio below 1.55, and at least one family member with a vitelliform lesion. ARB and ARB-like patients had bilateral multifocal vitelliform lesions with scattered lipofuscin in the posterior pole with subretinal fluid or cystoid edema, and reduced light rise with EOG. AVMD classification required age of onset older than 45, vitelliform foveal lesion between the ellipsoid layer and the RPE in a least one eye, and normal or only slightly abnormal EOG. All underwent genetic testing after diagnosis.
In total, 53 of the 134 patients had mutations of the BEST1 gene. Of the 87 patients diagnosed with BVMD, 39 had heterozygous mutations of the BEST1 gene with a 45% detection rate. BEST1 mutations were found in 14 of the 30 patients diagnosed with ARB and ARB-like dystrophy. Five of the ARB patients had compound heterozygous mutations, two had homozygous mutations and seven had heterozygous mutations. The mutation rate of detection for the ARB and ARB-like group was 82%. ARB-like patients are defined in this study as those patients with a single heterozygous mutation or without mutation. None of the 30 patients diagnosed with AVMD had mutations of the BEST1 gene. There were 37 different BEST1 mutations detected; five of those mutations were novel, the other 32 had already been reported. Interestingly no mutations in IMPG1, IMPG2, or PRPH2 genes were found in this cohort. Mutations in those genes are found to be linked to vitelliform macular dystrophies in the West.
Of particular interest, their results showed those with homozygous or compound heterozygous mutations had the shortest axial length, the shallowest anterior chamber, and the highest IOP. This study elucidated a trend that showed narrow anterior chamber associated with increasing number of BEST1 mutations. This paper demonstrated an association between BEST1 mutation and increased risk for angle closure glaucoma. As such, the authors suggested considering the risk for angle-closure glaucoma in all patients with BEST1 related dystrophies.
Xuan Y, Zhang Y, Zong Y, et al. The clinical features and genetic spectrum of a large cohort of chinese patients with vitelliform macular dystrophies. Am J Ophthalmol. 2020; Aug;216:69-79.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
Polypoidal Choroidal Vasculopathy: Clinical and OCT Features
Introduction
Polypoidal choroidal vasculopathy (PCV) is a condition clinically characterized by multiple, recurrent, serosanguineous pigment epithelial and neurosensory retinal detachments.1,2,3
PCV is caused by an abnormality of inner choroidal vessels secondary to pericyte abnormality and thinned out endothelial cells.1 The cause of such manifestations remains unclear.
Case Report
A 72-year-old male presented for an evaluation of decreased vision in both eyes for the past four years. His systemic history included diabetes mellitus type 2, hypertension, hypercholesterolemia and HIV. His medications included Lisinopril, Metformin, Glipizide, Hydrochlorothiazide, Ranitidine, Sustiva, Lipitor, Norvasc, and Flomax. The patient denied any family history of ocular conditions, including blindness. His unaided visual acuities in the distance were 20/40 OD and 20/50 OS and he was able to pinhole to 20/30 OD, OS. His manifest refraction was +1.00-0.75x055 OD and +1.25-0.75x105 OS with a +2.50 add. His best-corrected VA was 20/25 OD, OS after subjective refraction. All preliminary and anterior segment testing was unremarkable. The lenticular evaluation revealed 2+ nuclear sclerosis and 2+ cortical cataracts in both eyes. Intraocular pressure was 16, 17 mmHg OD, OS respectively.
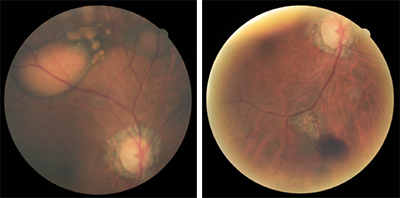
The posterior segment examination revealed multifocal pigment epithelial detachments with subretinal and intraretinal hemorrhaging superior and nasal in the right eye (Figure 1). The left eye revealed a large, hemorrhagic PED in the temporal macula without any evidence of subretinal fluid. The optic nerve and peripheral exam was unremarkable in both eyes. The patient was sent to the retinal specialist for evaluation and a diagnosis of PCV was confirmed. The patient was treated with a Lucentis injection in both eyes.
Discussion
The use of multimodal imaging including indocyanine green angiography (ICG), enhanced depth imaging (EDI), optical coherence tomography (OCT), optical coherence tomography angiography (OCTA), and fundus photography has greatly aided our ability to capture, study, and understand the pathophysiology of PCV. Some studies suggest PCV is a subtype of neovascular AMD; however, this is largely debated in the literature.1,2 There are two large studies that have well-established diagnostic criteria for PCV—the Japanese Study and the EVEREST Study.2 The Japanese study suggests a diagnosis of PCV when one or both of the following criteria are met: orange-red elevated lesions on fundus examination and/or characteristic polypoidal lesions seen on ICGA.2 The EVEREST study suggests a diagnosis of PCV with the presence of focal subretinal hyperfluorescence on confocal ICGA within the first six minutes plus 1 or more of the following criteria: nodular polyps on stereoscopic evaluation, hypofluorescent halo around nodules, presence of branching vascular network, pulsation of polyps on ICGA, orange subretinal polyps seen on fundus photography, or massive submacular hemorrhage(>4 disc diameters in size).2
The estimated prevalence of PCV in neovascular AMD is 8 to 10% and 41% in Japanese individuals.3 A recent study evaluated OCT features in Korean patients and developed an OCT-based diagnosis of PCV with high sensitivity and specificity.4 This study reported a diagnosis of PCV if three of more of the following findings were found on the OCT—multiple retinal pigment epithelial detachments (RPED), a sharp RPED peak, an RPED notch, a hyporeflective lumen representing polyps, and hyper-reflective intraretinal hard exudates.4 An OCT scan of our patient demonstrated many of the aforementioned characteristics (Figure 2). Other common OCT features include a double-layer sign, thickened choroid, and sometimes focal choroidal excavation.5
Conclusion
Indocyanine green angiography (ICGA) has traditionally been considered the gold standard used to diagnose PCV however, other, more readily available imaging modalities may aid in the diagnosis. In our case, fundus photography and OCT imaging helped document and illustrate features of PCV.
Figure 1 (top of newsletter)
Left image: Fundus photo of the right eye displaying a 3 to 4 disc diameter serosanguinous PED with corresponding RPE changes and smaller adjacent PEDs. A smaller serosanguinous PED can be seen superior nasal in the right eye. Right image: A large subretinal hemorrhage along the inferior temporal arcade with adjacent chorioretinal scarring associated with recalcitrant disease.
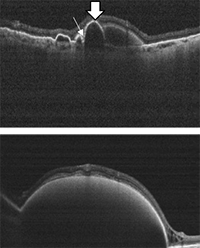 |
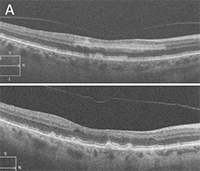
Figure 2
OCT image of the right eye showing a sharp retinal pigment epithelial detachment (RPED) peak (thick arrow) and an RPED notch (thin arrow).
OCT image of the large serosanguinous PED in the right eye with overlying intraretinal hard exudates. |
References:
1) Kumar A, Kumawat D, Sundar D, et al. Polypoidal choroidal vasculopathy: a comprehensive clinical update. Therapeutic Advances in Ophthalmology. 2019 Feb;11:1-26.
2) Chaikitmongkol V, Cheung C, Koizumi H, et al. Latest developments in polypoidal choroidal vasculopathy: epidemiology, etiology, diagnosis, and treatment. Asia-Pacific Journal of Ophthalmology. 2020 June;9(4):260-8
3) Acton J, Ogino K, Akagi Y, et al. Microperimetry and multimodal imaging in polypoidal choroidal vasculopathy. Scientific Reports. 2018 Oct; 8: 15769.
4) Chang Y, Kim JH, Kim JW, et al. Optical coherence tomography-based diagnosis of polypoidal choroidal vasculopathy in Korean Patients. Korean J Ophthalmology. 2016; June; 30(3):198-205
5) Cheung C, Lai T, Chen S, et al. Polypoidal choroidal vasculopathy. J ophthalmology. 2018 May. 125(5). |

IN THE
NEWS
 |
Connecting with Patients Through Off-Site Exams
Given the COVID-19 climate and the resulting push for remote patient care, Topcon is the latest to premiere an ocular telehealth software platform. The aptly named RDx connects to the Topcon’s digital phoropter which can then be controlled remotely by the practitioner. The system presets the starting point for refraction by importing the autorefractor and lensometer data. There is also an integrated face-to-face consultation dashboard. The company touts enhanced efficiency and work flexibility for those who use their remote platform. According to the Topcon RDx brochure, the list of connectable instruments continues to increase and includes slit lamp, visual fields, fundus camera and optical coherence tomography (OCT).
|
 |
Getting Your ELECTROLIGHTs
Diopsys recently announced a collaboration with LumiThera to evaluate the ability of Photobiomodulation (PBM) treatment for dry age-related macular degeneration (AMD) using the Valeda Light Delivery System (VLDS). PBM utilizes non-ionizing electromagnetic energy to initiate photochemical changes in photon-accepting tissues—mitochondria being a main target. Specifically, cytochrome C oxidase is a critical protein involved in regulation of mitochondrial activity. This protein is a key photoacceptor of light in the far red to near-infrared spectral range, according to the LumiThera website. Subjects in the trial will receive three PGM treatments per week for three weeks (nine total) and be followed for up to six months. Multi-focal ERG will be the protocol by which functional changes are measured.
|
 |
Bausch Plans to Il-Luminate Its AMD Product Pipeline
Bausch Health agreed to acquire and option to purchase all ophthalmology assets of Allegro Ophthalmics. This includes all global rights to risuteganib (Luminate®), which successfully met the primary endpoint in a Phase II clinical trial for intermediate AMD. Risuteganib regulates mitochondrial dysfunction and downregulates oxidative stress response to restore retinal homeostasis. Oxidative stress leads to increased vascular permeability, angiogenesis, inflammation, and cell death and neurodegeneration, according to company data. Phase III studies are pending. |
 |
On-label Bevacizumab May Be on the Outlook
Since the mid-2000s, retinal specialists have turned to compounded, off-label bevacizumab for the treatment of various angiogenic conditions. Over the years, stories periodically broke regarding contamination and/or inconsistency due to the nature of compounding. Now, Outlook Therapeutics—through a supplemental, open-label safety study—plans to evaluate bevacizumab-vikg (ONS-5010, Lytenava™) for the treatment of wet AMD. A fully enrolled Phase III trial should be completed early next year. Using both data sets the company hopes to file a Biologics License Application (BLA), making it the first bevacizumab-vikg to become on-label and giving clinicians an approved source of the drug free from compounding. Along with vials, single-use prefilled syringes will be available. Outlook plans to also seek approval for diabetic macular edema (DME) and branch retinal vein occlusion (BRVO).
|
 |
A “One-and-Done” Intravitreal Injection?
Interim Phase I data is out on the clinical trial of ADVM-022 intravitreal injection gene therapy for wet AMD—and the news is good. Adverum Biotechnologies’ OPTIC trial reported drug durability out to 92 weeks following a single intravitreal injection. ADVM-022 maintained both BCVA and CRT, and in some cases improved the latter. Supplemental anti-VEGF injections occurred in only one out of 15 high-dose patients, which equated to a 99% reduction in treatment burden. One-third of the low dose group received anti-VEGF injections. Treatment appears safe with no reports of intraocular inflammation.
|
 |
It’s in the Genes
While ADVM-022 targets wet AMD, Gyroscope Therapeutics is in Phase II of their gene therapy agent (GT005) for people with geographical atrophy (GA) secondary to dry AMD. GT005 is a one-time adeno-associated virus (AAV) based gene therapy designed to restore the balance to an overactive complement system by increasing production of Complement Factor I. Most patients with GA carry at least one genetic variant in a complement gene. The HORIZON trial will analyze the drug’s effectiveness at slowing the progression of GA.
|
 |
A LION Without a Leash
Norlase recently announced the launch of a head-borne green laser photocoagulator dubbed the LION. Fully integrated into a Keeler indirect ophthalmoscope, the LION has no fiber tether. A battery powers the unit which utilizes an advanced wireless interface with voice control of parameters. The LION eliminates the need for an external laser console since the green laser is integrated into the device. |

|
IMAGE QUIZ ANSWER
The correct answer, image A, shows acute paracentral middle maculopathy (PAMM), which is caused by ischemia in the retinal intermediate/deep capillary plexis. PAMM can occur isolated or in conjunction with retinal vasculature disease such as diabetic retinopathy, vascular occlusion or sickle cell disease. Acute PAMM lesions on clinical exam appear gray or white. This corresponds on OCT to a hyper-reflective band located at the junction of the outer plexiform layer (OPL) and inner nuclear layer (INL) as noted in the top image.
After a few weeks, a PAMM lesion can no longer be appreciated on clinical exam. At that point, OCT shows atrophy and thinning of the INL which is seen here in the bottom image.
B: ERM with lamellar hole C: foval VMT with ERM D: active APMPPE lesion
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Meret Thomas-Huebner, OD
Contributor
Jim Williamson, OD, FAAO
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|