| |
Volume 15, Number 4 |
December 2019 |
|
|
Inside
This Issue |
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
In the modern internet era, our resources and tools for learning have drastically changed. Now the few textbooks that I still own are carrying a thick layer of dust! This has many advantages, as resources are quickly available. But unfortunately, it is also easy to fall into the Google search trap of finding poorly researched or incorrect information. So I’d like to share a new resource that I recently learned about for all things retina: Retina Rocks Aptly named, as all of us here at ORS love all things retina. The site contains thousands of images and journal references for many retinal conditions from AMD to Zika retinopathy. The site is free to use and only requires creating an account. You are encouraged to share images to the creators to collaborate and expand the image database. Check it out! Having quality online resources to turn to is critical. Many thanks to the website’s creators (Steven Bloom, MD; Leon Nguyen, OD; and Inder Singal, MD) for sharing this educational resource.
On a final note, I recently got back from the 2019 ORS Retina Update, hosted in collaboration with the Review group. This year’s meeting did not disappoint. And that wasn’t just because I was a speaker. Thanks to our program chairs, Drs. Mohammad Rafieetary and Steve Ferrucci, for putting on another great meeting. Plans are already underway for Retina Update 2020, tentatively set for early December. Mark your calenders to get down to sunny Scottsdale.
Happy New Year!
Anna Bedwell, OD, FAAO
Editor-in-Chief
PRESIDENT'S MESSAGE
As 2019 comes to a close, it’s good to reflect on the past and plan for the future. The ORS welcomed two new fellows this year: Dr. Marlon Demerit and Dr. Raman Bhakhri. We are always looking for and are open to new applicants to fellowship in the ORS. Any interested OD should visit our website and search the Join Us section for information. We are happy to help anyone with the application process.
Our annual Retina Update meeting that was recently held in Scottsdale, Arizona, Dec. 5 to 6 was a tremendous success. The program was outstanding, and the attendance increased by more than 50% from 2018. We had over 170 doctors in attendance, with many sponsors and exhibitors. The annual Larry Alexander Legacy Lecture on macular disease was provided by a powerful combination of Anna Bedwell, OD, Stephanie Cooper, OD, and Mary Beth Yackey, OD. All other speakers were wonderful as well. We are planning on the 2020 Retina Update being held at the same time of year and hopefully at the same location so please mark your calendars and plan on attending.
The ORS website has also seen some improvements this year. It was updated to a more modern design, and just this week we added an image gallery for ORS fellows to share photos to assist in the educational goals of the ORS.
As we move forward into 2020, my wish, and our goal as the ORS, is to continue to strive to accomplish our mission of advancing vitreo-retinal knowledge of all optometric providers and students. I hope all of us will be active in this vital endeavor. Have a wonderful holiday season and a fantastic 2020.
Jeffrey Austin, OD, FAAO
ORS President

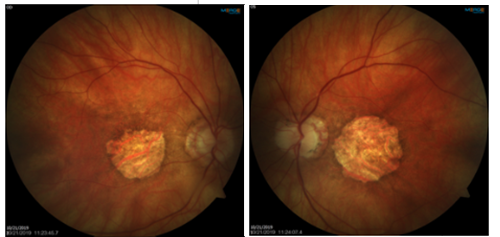
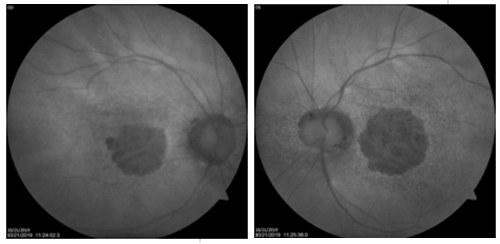
YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.
Answer appears later in newsletter.

Image Gallery
Which of the following images represents vitreomacular traction?
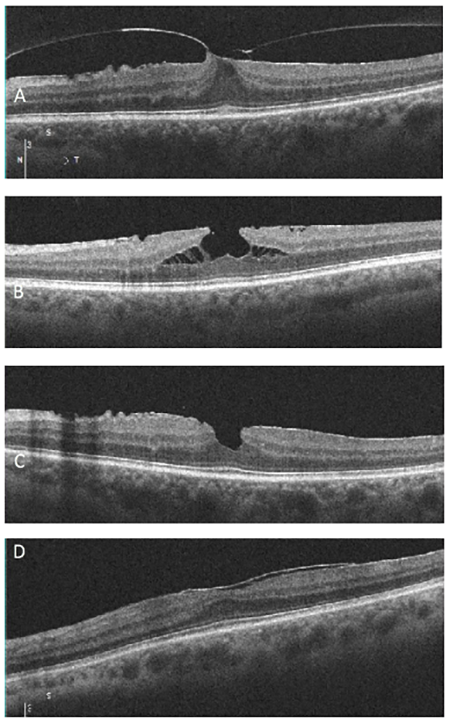
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Retinal Vein Occlusion is Associated with Low Blood High-Density Lipoprotein Cholesterol: A Nationwide Cohort Study
This review article from Korea examined the factors associated with retinal vein occlusions in a Korean population. Data from the Korean National Health Insurance Service was used to look at over 117,000 patients who had suffered a retinal vein occlusion, either CRVO or BRVO. Not surprisingly, compared to Koreans in the registry who had not experienced a retinal vein occlusion, those who had were much more likely to suffer from many cardiovascular conditions. They had higher BMI, increased waist circumference, increased fasting blood glucose, and increased blood pressure (both systolic and diastolic). In addition, they had increased LDL cholesterol, increased triglycerides, and increased total cholesterol. This study also reported for the first time that decreased HDL cholesterol levels were associated with retinal vein occlusions. It is possible that these findings may not be fully applicable in patients of other races and ethnicity, but the very large number of patients included in this study certainly gives it credence.
Kim J, Lim DH, Han K, et.al. Retinal vein occlusion is associated with low blood high-density lipoprotein cholesterol: a nationwide cohort study. Am J Ophthalmol. 2019;Sep;205:35-42.
Posterior Fundus Hemorrhages: Frequency and Associated Factors. The Beijing Eye Study.
This article discussed the prevalence of retinal hemorrhages in the Beijing Eye Study population, ages 50 and over. The review produced some very interesting statistics. Hemorrhages were categorized as being flame-shaped, dot-like, or areolar/rounded. They were also grouped by locations, which were optic disc, peripapillary, macular, and peripheral. Gradable fundus photographs were used to determine the presence or absence of hemorrhages; 6,798 eyes of 3,417 people had useable photos. Overall, hemorrhages were found in 7.6% of eyes and 12.7% of people. Diabetes was believed to be the cause of 36.7% of these hemorrhages, retinal vein occlusions 12.6%, PVD 4.5%, glaucoma 2.7%, AMD 1.6%, and hypertensive retinopathy 1.9%. Cotton wool sports were also present in 11.3% of eyes with hemorrhages. Interestingly, there was no immediately identifiable cause for the retinal hemorrhage or hemorrhages in 39.6% of eyes. Regarding location, 9.5% of the hemorrhages were at the optic disk, 37.7% were peripapilllary (within two disc diameters of the disc but not touching it), 24.3% were macular, and 6.2% were peripheral. The hemorrhages were found in two or more of these locations in 22.3% of eyes. Flame shaped hemorrhages were seen in 73.2% of eyes with hemorrhages, dot hemorrhages in 25%, and rounded in 1.6%.
While these statistics provide valuable information on the prevalence of retinal hemorrhages in a large group of people, it is important to keep in mind the relatively homogenous nature of the population studied. These values may not be fully applicable to other populations.
Zhou JQ, Wang YX, Xu L et al. Posterior fundus hemorrhages: frequency and associated factors. The Beijing Eye Study. Retina. 2019;Jun;39(6):1206-15.
Comparison of Ranibizumab Alone vs. Ranibizumab with Targeted Retinal Laser for Branch Retinal Vein Occlusion with Macular Edema
Anti-VEGF injection is the present gold standard therapy for macular edema as a result of retinal vein occlusion. But even with anti-VEGF treatment, macular edema can recur with the need for repeated injections. This study explored an alternative by using ultra-widefield (UWF) imaging for targeted retinal photocoagulation (TRP) for capillary non-perfusion. UWF imaging captures the posterior pole out to the peripheral retina at a 200-degree field.
This was a prospective, randomized trial at a tertiary eye care center evaluating 33 eyes of 32 patients with branch retinal vein occlusion (BRVO) and macular edema. All patients had a visual acuity of between 20/63 and 20/400, and central subfoveal thickness greater than 300 microns. One group received 0.5 ranibizumab only while the other group received 0.5 ranibizumab with UWFFA guided TRP. Both groups were treated with three loading injections at monthly follow up and then prn. For the second group, UWFFA-guided TRP was performed one week after the first injection. TRP was applied to the areas of retinal capillary non-perfusion and junction between ischemic and nonischemic retina.
Both groups had similar increases in VA, substantial decrease in CST and similar improvement in contrast sensitivity. In the injection-only group, the number of injections necessary ranged from three to eight (average 5.76) compared to the injection + TRP group with three to six (average 4.06) injections. This was statistically significant (p<0.001).
The study group postulated that untreated retinal nonperfusion may play a role in VEGF production, causing recurrence of macular edema in BRVO. The goal is to target areas of nonperfusion to ultimately decrease VEGF production and for patients to decrease the number of anti-VEGF injections. This study, though small, supported that TRP in combination with anti-VEGF could be used to decrease recurrence of macular edema in BRVO.
Goel S, Kumar A, Ravani RD, et al. Comparison of ranibizumab alone versus ranibizumab with targeted retinal laser for branch retinal vein occlusion with macular edema. Indian J Ophthalmol. 2019;67:1105-8.
Prevalence of Resolved Paracentral Acute Middle Maculopathy Lesions in Fellow Eyes of Patients with Unilateral Retinal Vein Occlusion
Paracentral acute middle maculopathy (PAMM) represents deep retinal ischemia and has been found in a number of vascular diseases of the retina including retinal vein and arterial occlusions, sickle cell retinopathy, and Purtscher retinopathy. Acute PAMM lesions are visible on OCTA as signal voids in the deep capillary plexus. However, as these lesions resolve, reperfusion can occur and be missed with OCTA. More accurately, resolved PAMM lesions can be identified as inner nuclear layer thinning with outer plexiform layer elevation on cross-sectional OCT scans.
This case-control observational study used this definition of resolved PAMM lesions to compare patients with unilateral CRVOs and BRVOs to age-matched healthy individuals. They found that PAMM lesions were present in 71.1% of fellow eyes of patients with BRVOs, 71.4% of those with CRVOs, and only 19.3% of eyes of healthy individuals. Of the healthy volunteers, PAMM lesions were bilateral in 72.7% and were associated with arterial hypertension. This study also compared the size of PAMM lesions, with small resolved lesions less than 300 microns of elevation of the outer plexiform layer and large lesions greater than 300 microns. Eleven percent of healthy eyes had large PAMM lesions, while 25% and 40% of fellow eyes with BRVOs and CRVOs, respectively, had large PAMM lesions.
This study concluded that although resolved PAMM lesions can be found in healthy individuals, they are much more prevalent in fellow eyes of RVO patients and may be considered as a finding associated with the risk of an RVO.
Maltsev DS, Kulikov AN, Burnasheva MA, et al. Prevalence of resolved paracentral acute middle maculopathy lesions in fellow eyes of patients with unilateral retinal vein occlusion. Acta Ophthalmol. 2019;Jul 25. [Epub ahead of print].
Predictors of Neovascular Glaucoma in Central Retinal Vein Occlusion
Central retinal vein occlusions can be divided into ischemic or nonischemic categories. Nonischemic CRVOs make up about 80% of cases and are less likely to develop neovascular glaucoma (NVG) than ischemic CRVOs. The Central Vein Occlusion Study defined an ischemic CRVO as greater than or equal to 10 disc diameters of nonperfusion on fluorescein angiography. However, determining if a CRVO is ischemic or nonischemic with FA can be difficult, as many patients present with dense intraretinal hemorrhaging. This study reviewed 646 patients with an acute CRVO that had to be present for less than 90 days, who lacked anterior segment neovascularization, and had no previous intravitreal anti-VEGF injection to determine risk factors for NVG. They found those with a worse initial visual acuity, relative RAPD, and systemic disease of hypertension were significantly more at risk. Of note, almost half of patients that developed NVG had a VA of better than 20/400, and those with a VA of 20/50 or better were less likely to develop NVG. Additionally, an RAPD was found to at least double the concern that an eye would develop NVG. This study also found that 13% of patients developed NVG, and the average time to development was about 421 days, ranging from 21 to 1,286. Patients who received anti-VEGF treatment for macular edema throughout the treatment time were still at risk for NVG, with an average development time of 212 days after their last injection. This is important for two reasons: one, because patients are at risk for NVG past the typical “90-day glaucoma” timeline that is clinically taught, and those receiving anti-VEGF treatments are still at risk as well; and two, unfortunately, previous anti-VEGF injections do not prevent NVG from developing.
Although the authors of this study acknowledged their limitations, this study has received some criticism. Drs. Calugaru and Calugaru responded that gonioscopy should have been performed at each visit to catch early neovascularization. Although this would have been an ideal, the retrospective design of the study made this impossible. Critics also took issue with the fact that this study did not better divide patients into ischemic and non-ischemic CRVOs. However, the goal of this study was to determine risk factors that would be easy to account for on a routine exam, without the need for angiography, electroretinography, or Goldmann visual field testing. Finally, they questioned the anti-VEGF treatment, noting that the agent used, dosage, and treatment timeline should have been better documented before concluding that anti-VEGF treatments do not prevent NVG from developing. Drs. Kumawat, Sahay, and Shah agreed with this issue as well. Additionally, they commented on glaucoma being a risk factor for NVG and felt that history may have been lacking to rule out preexisting and undiagnosed glaucoma. As a final point, they argued that entering visual acuity may have been confounded by the presence of macular edema and that VA after resolution of macular edema may be more predictive of the risk of NVG.
Rong AJ, Swaminathan SS, Vanner EA, et al. Predictors of Neovascular Glaucoma in Central Retinal Vein Occlusion. Am J Opthalmol 2019; 204:62-69.
Dan Călugăru, Mihai Călugăru. Comment on: Predictors of Neovascular Glaucoma in Central Retinal Vein Occlusion. Am J Opthalmol 2019; 205:200-01.
Devesh Kumawat, Pranita Sahay, Pooja Shah. Comment on: Predictors of Neovascular Glaucoma in Central Retinal Vein Occlusion. Am J Opthalmol 2020; 209:217-18.
Thirteen Years of Intravitreal Anti-vascular Endothelial Growth Factor Therapy: the Promises and Burdens of a Paradigm Shift Told from the Perspective of the Largest Retina Service in Norway.
Use of anti-VEGF therapy has quickly risen since its introduction over a decade ago. This study looked at the impact of anti-VEGF at Oslo University Hospital over the course of 13 years of use (2006 to 2018).
The data included 7,984 individual patients and 153,823 total injections. Patients receiving injections went from 130 in 2006 to 3428 (940 new patients and 2,488 carrying over treatment from the prior year) in 2018. As many patients were chronically receiving treatment on average seven injections per year, the yearly number of episodes of care skyrocketed from 224 in 2006 to 22,029 in 2018. Not surprisingly, given the population in Norway, the most common diagnosis coded in the study was H35.3, degeneration of macula and posterior pole, at 69% of total injections. But H35.5 showed a decreasing proportion over time, whereas diabetic retinopathy (H36.0) and vein occlusion (H34.8) demonstrated an increase. The selection of anti-VEGF agent also changed over time. By 2018, 49% of injections were with bevacizumab, 46% with aflibercept, 4% with ranibizumab, and 1% with dexamethasone implants. Once aflibercept was approved in 2013, the number of aflibercept injections quickly rose, even considering that it was used at this hospital for treatment resistant cases.
As expected, this study found a substantial increase in anti-VEGF injections. But it also showed a steady rise in aflibercept therapy to nearly a 1:1 ratio with the much more cost-effective bevacizumab, which recognizes the ever-increasing cost burden on healthcare systems.
Jørstad ØK, Steffensen LA, Eriksen K, et al. Thirteen years of intravitreal anti-vascular endothelial growth factor therapy: the promises and burdens of a paradigm shift told from the perspective of the largest retina service in Norway. Acta Ophthalmol. 2019 Jul 1.[Epub ahead of print]

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
A 67-year old Caucasian female presented for an annual exam, hoping to get an updated glasses prescription. Her last eye exam had been a year ago, and at that time she was being followed for dry macular degeneration OU and was taking AREDS 2 supplements. She had stopped going to regular follow-ups with her previous doctor because her vision was not changing. Her medical history was remarkable for hypertension and hypercholesteremia; she was a former smoker.
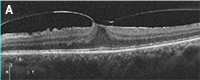
Best-corrected visual acuity was 20/200 OD, and 20/400 OS. Dilated fundus examination and fundus photography revealed a well-circumscribed area of chorioretinal atrophy at the macula, OD and OS. Fundus autofluorescence showed a complete absence of signal in the area of atrophy. SD-OCT (see figure 1 below) showed absence of the outer retinal layers. No drusen were visible on DFE, fundus photography, or SD-OCT.
The differential diagnosis for this case included central areolar choroidal dystrophy or geographic atrophy from age-related macular degeneration. Given the similar retinal findings, these two diseases are easily confused, especially since up to a third of CACD patients develop vision loss at an older age and physical findings can vary greatly between patients.1
CACD is an inherited disorder most often caused by an autosomal-dominant mutation in the peripherin/RDS gene.2 In the earliest stage of CACD, retinal signs include subtle mottling of the posterior pole with speckled areas of hyperfluorescence on FAF and RPE disruption on SD-OCT. Ultimately, these changes progress to a well-circumscribed round or oval area of chorioretinal atrophy at the center of the macula.1 Patients become symptomatic in the third to fourth decade of life, with a dramatic decline in central visual acuity occurring between the fourth and seventh decade. ARMD is a multifactorial disease characterized by the presence of drusen.2 In the advanced, non-exudative form, geography atrophy of the photoreceptors, RPE, and choriocapillaris leads to profound central vision loss. In contrast to the sharply demarcated atrophy visible in CACD, fundus photography and FAF in ARMD patients show irregular and/or branching atrophy that often extends beyond the central macula.1 In addition, drusen can be visualized with SD-OCT and surrounding the areas of atrophy with FAF.
Based on the well-circumscribed area of atrophy and lack of drusen, the patient was diagnosed with central areolar choroidal dystrophy. Although visual prognosis is poor, it is important to differentiate CACD from ARMD because of its autosomal-dominant inheritance. Interestingly, this patient only knew of a grandfather with a history of vision loss, but she was educated on the inheritance pattern and encouraged to inform family members of the importance of obtaining baseline eye exams. Finally, she was instructed to discontinue AREDS 2 supplementation, as there is no proven treatment for CACD. She was set up for a low-vision evaluation and will continue to be monitored annually.
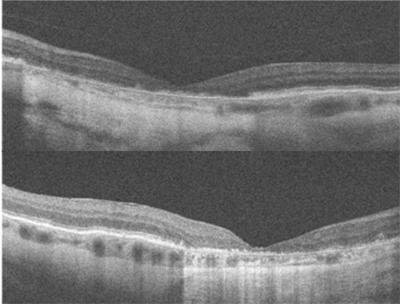 |
| Figure 1: OCT of the macula in the right eye (left image) and left eye (right image) showing loss of the outer retinal layers. |
Larissa Krenk, OD
Primary Care Resident
Indianapolis Eye Care Center
Indiana University School of Optometry
1. Smailhodzic, D., et al. Central areolar choroidal dystrophy (CACD) and age-related macular degeneration (AMD): differentiating characteristics in multimodal imaging. Investigative Ophthalmology & Visual Science. 2011;52:8908-18.
2. Rodman, J. , et al. (2013). Central areolar choroidal dystrophy with dominant drusen. Journal of Optometry. 2013;6(2):114-22.
|

IN THE
NEWS
 |
New FDA Warnings Include AMD and Cataracts
The FDA recently proposed 13 cigarette health warnings that use 12 statements. Two of these statements are eye-related:
WARNING: Smoking causes age-related macular degeneration,
which can lead to blindness.
WARNING: Smoking causes cataracts, which can lead to blindness.
Each text warning is paired with a photo. The cataract warning shows an external facial photograph of a dense unilateral opacity, while the AMD one depicts the close-up of an eye receiving an anti-VEGF injection. Comments on the suggested warnings closed on October 15. Interestingly, the FDA used an African-American male to depict an intravitreal injection from AMD.
|
 |
Longer Acting Anti-VEGF Approved
If only they would approve a drug we all knew how to pronounce. Novartis announced in October the FDA’s nod of its anti-VEGF agent Beovu (brolucizumab-dbll). Based on the Phase III HAWK and HARRIER clinical trials, Beovu touts greater fluid reduction vs. aflibercept along with a three-month injection interval after an initial loading dose. Most common adverse reactions were similar to the control aflibercept, except for intraocular inflammation (4% Beovu vs. 1% control).
|
 |
Drug Shows Promise for Geographic Atrophy Treatment
Patients with geographic atrophy secondary to AMD may once again have hope for a treatment option given Iveric bio, Inc.’s announcement of positive results from a Phase IIb randomized, double masked, sham controlled Zimura® clinical trial involving 286 dry AMD patients. The drug is a complement factor C5 inhibitor designed to reduce the rate of retinal tissue degeneration. Patients treated with 2mg and 4mg Zimura® demonstrated a nearly 30% reduction in the mean rate of GA over 12 months compared with the sham control group. Phase III could be the key, as a previous agent—lampalizumab—failed during this stage of the approval process.
|
 |
Long-term Pentosan Polysulfate Sodium Use Tied to Macular Disease
Elmiron®—the only FDA-approved treatment for interstitial cystitis or bladder pain—has been shown to cause retinal damage that may mimic a pattern dystrophy or AMD, according to researchers. The study, which was presented at the 2019 American Academy of Ophthalmology annual meeting, revealed a link between pentosane polysulfate and maculopathy. Additionally, observed toxicity rose with the amount of drug consumed. Investigators noted the rate of toxicity of those taking 500 to 1,000 grams was 11%, and that number jumped to 42% when patients were taking 1,500 grams or more. The authors suggested annual ophthalmic screenings for these patients and communication with the urologist regarding any retinal abnormality attributed to drug toxicity.
|
 |
Contact Lens Approved for Myopia Management
The FDA recently approved Coopervision’s MiSight® 1 day contact lens for myopia management. The studies enrolled 144 myopic children ages eight to 12 from Singapore, Canada, the United Kingdom, and Portugal, which accounts for the FDA’s approved age range for initial fitting. Slowed myopia progression was noted in 59% measured with cycloplegic spherical equivalent and 52% as measured by axial length. Given the incidence of myopic maculopathy and other related conditions, it will be interesting to see the follow up data.
|
 |
Niacin-induced Cystoid Maculopathy Reversible
A report from retinal specialists from the New York Eye and Ear Infirmary of Mount Sinai showed that niacin-induced cystoid maculopathy is reversible. Niacin can be purchased over-the-counter or in a prescription form. It is used for lowering hyperlipidemia or cholesterol. The findings included a case presentation of a patient taking six grams of niacin per day—well beyond the standard dose of one to three grams. Multifocal electroretinography detected the toxicity occurred with the Müller cells.
|
 |
A Tough Pill to Swallow
Bausch + Lomb recently introduced Preservision AREDS 2 formula minigel eye vitamins. These will replace the current softgel version and offer patients an easier-to-swallow option. The 120-count bottle has a suggested retail price of $32.99.
|
 |
Optos Widens Its Device Platform
Optos recently unveiled its newest device, Silverstone. This combined ultra-widefield retinal imaging device and UWF-Guided 100k cycles/second swept-source OCT (SS-OCT) captures 200-degree retinal images and 23 mm SS-OCT images. The longer 1,050 nm wavelength penetrates deeper (up to 2.5mm) and will allow enhanced choroidal imaging. Axial resolution is <7 um while transverse is <20 um.
|
| |
| |
|

|
IMAGE QUIZ ANSWER
The correct answer, image A, demonstrates vitreomacular traction, a complication of anomalous posterior vitreous detachment. There is partial perifoveal detachment of the vitreous cortex with the remaining attachment causing traction on the fovea. This traction results in anatomical changes to the retina such as loss of foveal contour (as seen in image A), pseudocyst formation, CME, retinal thickening, etc. The traction may release on its own or in 5 to 12% can progress on to full thickness macular hole. Symptomatic VMT can be treated with vitrectomy or ocriplasmin injection.
B: ERM with lamellar hole
C: ERM with pseudohole
D: ERM
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
SPONSOR NEWS

Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Larissa Krenk, OD
Contributor
Jim Williamson, OD, FAAO
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|