The incidence of drug-related keratitis has been increasing annually since 2004, hitting a peak in 2023. Keratitis can occur after prolonged use of ophthalmic or systemic drugs, often triggered by the direct damaging effects of a medication on the cornea or by the resulting reduced immune barrier function. In order to prevent this disease, identifying which drugs carry the most risk could guide treatment plans. A new study published in Translational Vision Science & Technology aimed to do just this, and revealed that many common ophthalmic medications used to treat glaucoma are not only some of the most potentially toxic but have a longer drug-induced time, typically exceeding 100 days.
Researchers used data from the FDA Adverse Event Reporting System (FAERS) spanning from Jan. 1, 2004 to Dec. 31, 2023. A total of 7,176 subjects were reported to have experienced drug-related keratitis adverse reactions, the age of which was approximately 56.2 ±20.3 years, with women comprising a slight majority at 52%. Over the course of the nearly 20-year period, women consistently reported a significantly higher incidence than males annually.
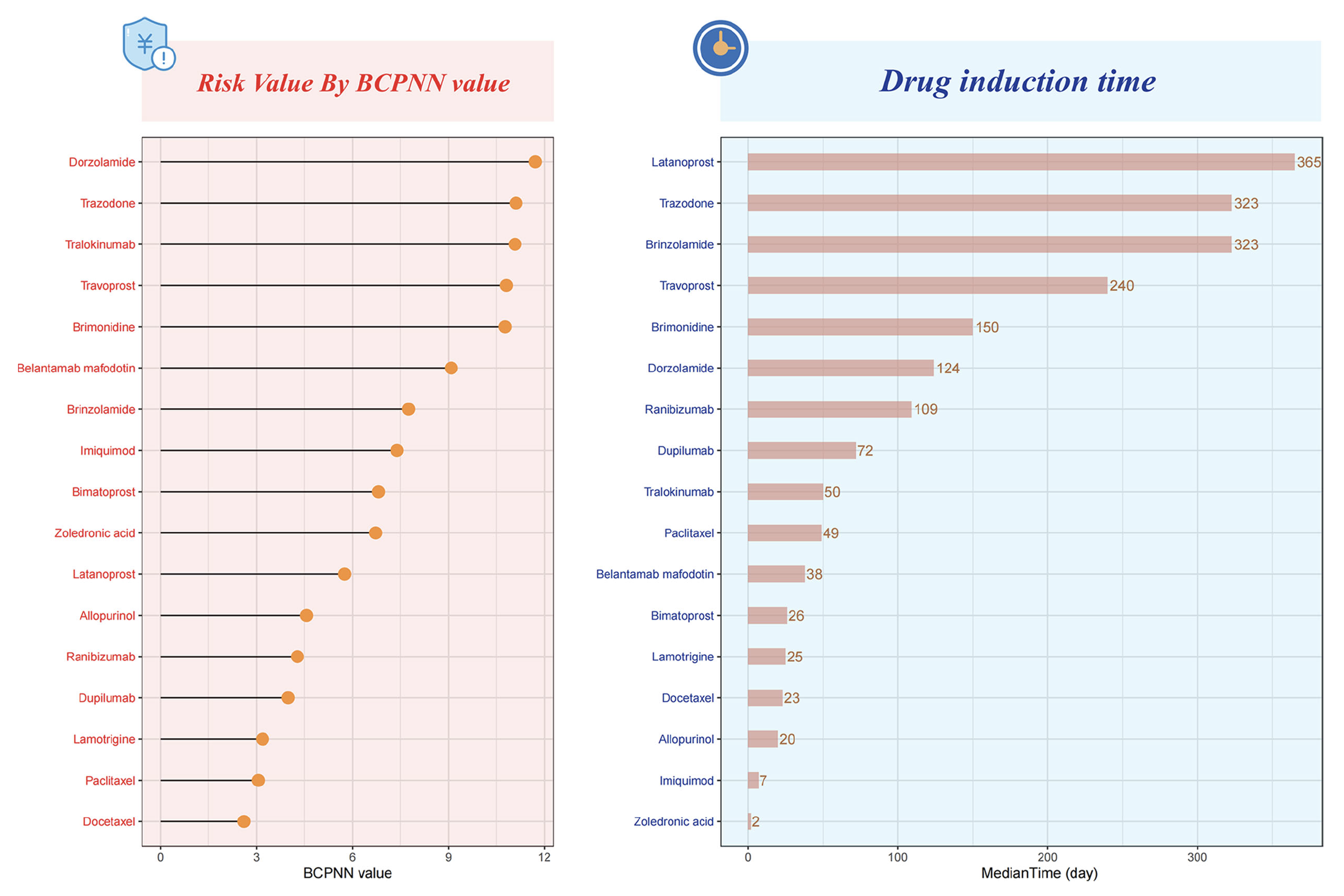
Ultimately, the study identified 17 drugs associated with drug-related keratitis, seven of which were ophthalmic medications. The top three ophthalmic drugs were dorzolamide, travoprost and brimonidine, followed by brinzolamide, bimatoprost, latanoprost and ranibizumab.
 |
|
A recent study published in Translational Vision Science & Technology reveals a significant increase in drug-related keratitis, particularly linked to common glaucoma medications such as dorzolamide, travoprost and brimonidine. Analyzing nearly two decades of FDA data, researchers also found that females are disproportionately affected. The study calls for increased awareness among clinicians to accurately diagnose and manage these adverse effects. These charts from the study show the distribution of risks and drug induction times for drug-related keratitis, arranged in descending order. Photo: Wu SN, et al. Transl Vis Sci Technol. September 17, 2024. Click image to enlarge. |
Using a data mining technique called Bayesian confidence propagation neural network, the researchers estimated that each of these raises the risk of keratitis roughly 11-fold under certain circumstances involving long-term use. The top three non-ophthalmic drugs were tralokinumab, trazodone and belantamab mafodotin.
“The potential ocular toxicity of medications used to treat glaucoma has been confirmed in several studies,” wrote the authors. According to previous research, the authors said, “Long-term use of antiglaucoma medications can lead to decreased tear secretion, decreased conjunctival goblet cells, and increased macrophages and lymphocytes, thereby affecting the normal function of the corneal epithelium. Furthermore, the topical use of prostaglandin derivatives may increase the risk of recurrence of herpetic keratitis.”
Prostaglandin analogs and carbonic anhydrase inhibitors were found to have a longer drug-induced time, which the authors say could be due to the prolonged treatment cycle associated with glaucoma-related drugs. “Due to the lack of a gold standard for diagnosing drug-related keratitis, it is challenging to differentiate it from exacerbation of primary ocular diseases or complications following ocular surface or intraocular surgeries,” they wrote in their paper for TVST. “Consequently, clinicians may mistakenly interpret corneal lesions during medication as worsening of the underlying eye condition, leading to increased dosage or prolonged medication duration, thereby exacerbating the condition.”
Clinicians should elevate their diagnostic awareness of drug-related keratitis to improve early detection rates and minimize lesion damage, the authors continued. “When prescribing medications to high-risk patients susceptible to drug-related keratitis, it is crucial to prevent the toxic effects of eye drops and the ocular disease risks associated with systemic medications,” they said. “The most important aspect is to accurately diagnose and treat the primary disease comprehensively, avoiding the indiscriminate mixing and abuse of multiple medications and minimizing the frequency and duration of medication use.”
Limitations identified in this paper include the lack of establishing a definitive causal relationship between targeted drugs and adverse reactions, as well as the use of FAERS, which relies on voluntary and spontaneous reporting and could introduce bias. They also point out that the modes of drug administration in FAERS aren’t specified, so it’s unclear if the term “ophthalmic” included topical eye drops or also encompasses periocular injections.
“By comparing and analyzing the differences in drug-induced time between ophthalmic and non-ophthalmic medications, it revealed important epidemiological characteristics of drug-related keratitis,” the authors concluded. “This provides valuable data-driven guidance for reducing the incidence of drug-related keratitis and guiding clinical medication… [T]his study lays the foundation for strengthening clinical decision-making and patient care in the context of medication-related ocular complications.”
| Click here for journal source. |
Wu SN, Chen XD, Zhang QH, et al. Drug-related keratitis: A real-world FDA Adverse Event Reporting System Database study. Transl Vis Sci Technol. September 17, 2024. [Epub ahead of print]. |


