Annual Cornea ReportFollow the links below to read other articles from our annual Cornea Report: An OD’s Guide to Corneal Transplant Options |
For many eye care providers and patients, keratoconus (KCN) management can feel like maintaining an undesirable status quo. Due to advancements in specialty contact lens technologies, corneal grafts are now only necessary for 10% to 20% of KCN patients.1 Notwithstanding, these patients still scored similarly to those with advanced macular degeneration on the National Eye Institute’s visual function questionnaire in CLEK Study (Collaborative Longitudinal Evaluation of Keratoconus Study).2-5 Another report by the same group found that self-perceived quality-of-life scores for KCN patients continue to decline over time.6 With postulated KCN prevalence reaching one in every 375 individuals, disease stabilization and quality of life improvement or maintenance are top priorities.7
Since its development in 2003, corneal crosslinking (CXL) has quickly become the treatment of choice for KCN progression control.8 Although CXL only received US Food and Drug Administration (FDA) approval in 2016 (Avedro’s KXL System and two photoenhancers, Photrexa and Photrexa viscous), we have been able to offer CXL treatments to patients for many years at Wills Eye Hospital under the auspices of clinical trials. As a result, we comanage many of these patients with community clinicians.
As with any new treatment procedure, a learning curve exists for clinicians to refine patient education and selection process, as well as other perioperative management-related protocols. An open channel of communication allows our Corneal Service to help comanaging clinicians to gain clinical comfort with CXL in their KCN practices. Here are 12 common questions our partner doctors ask; the answers can help you decide on how to best educate your KCN patients on CXL.
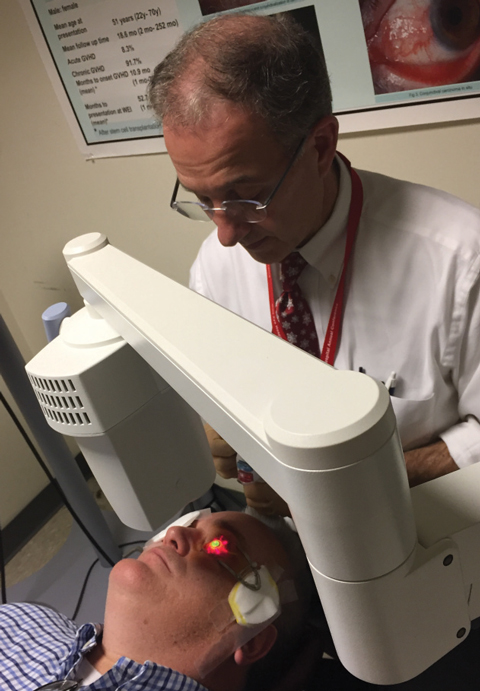 |
| Fig. 1. Christopher Rapuano, MD, performs standard a corneal crosslinking protocol with the FDA-approved KXL System. |
1. What is CXL and how does it work?
Crosslinking is a polymerization process that rearranges monomers into a three-dimensional network of polymers to increase the soundness of a molecular structure. This process naturally occurs in our bodies as connective tissues gradually stiffen over time. Facilitated by the endogenous enzyme lysyl oxidase in launching the required oxidative reactions, additional covalent bonds (or tissue “crosslinks”) are formed between and within collagen fibrils—yielding increased tissue biomechanical strength.9
Typically, the cumulative effects of natural crosslinking reactions are slow to manifest. In the late 1990s, researchers from the University of Dresden in Germany determined that the photochemical induction process was the most clinically viable method for boosting induction of crosslinks in the cornea, bringing about CXL.8 This study used 0.1% riboflavin (with 20% dextran in solution) as the photosensitizer to absorb a carefully calibrated ultraviolet (UV) energy dose, thus converting available tissue oxygen into singlet oxygen molecules. The resultant reactive oxygen species possesses sufficient energy to activate the lysyl oxidase enzymatic pathway, leading to formation of new covalent bonds within the corneal stroma.
The study from Dresden reported that all of the 23 progressive KCN eyes treated were stabilized, with 70% showing maximal keratometry flattening by 2.01D. Since then, many studies have achieved similar efficacy with good safety profiles in KCN patients using the same CXL protocol involving epithelial removal (Figure 1).10-13
 |
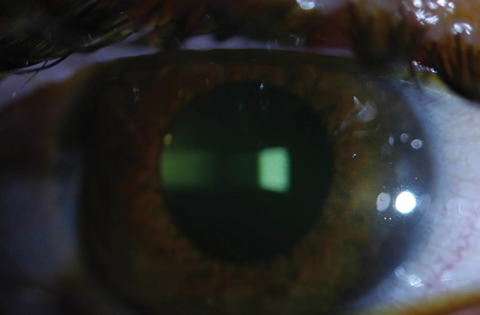 |
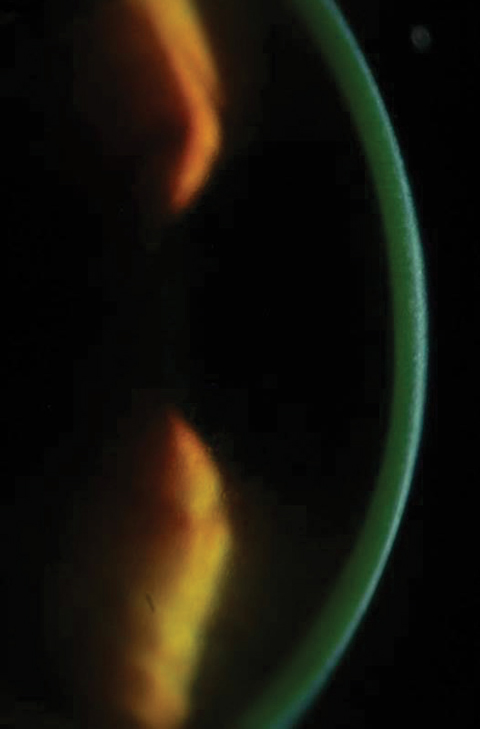
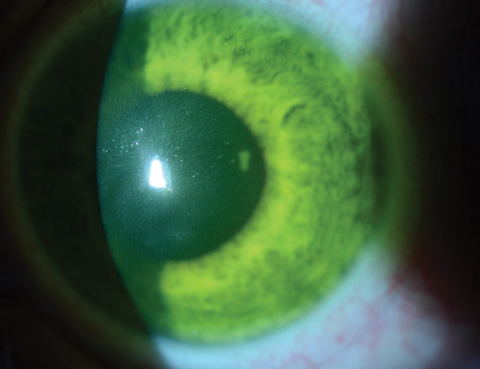
| Figs. 2a and 2b. Above, saturation of riboflavin seen in the corneal stroma after riboflavin loading. Below, after 30 minutes of riboflavin loading at two-minute intervals, clinicians must check for aqueous riboflavin staining. Click bottom image to enlarge. |
2. What is riboflavin’s role during CXL?
Since the bioavailable oxygen molecules in the cornea cannot be activated by UV light directly, a photosensitizing substance must act as an intermediate agent. Riboflavin catalyzes CXL’s photochemical reactions by transferring UV energy (specifically, UVA from 365nm to 370nm) to stromal oxygen molecules, thereby converting stable oxygen molecules into a more reactive singlet form. These reactive oxygen species then initiate intrastromal oxidative reactions.
Assuming UV energy is not the limiting resource, continuous oxygen replenishment and active riboflavin molecules are essential in maintaining the energy transfer required to perpetuate the CXL process.
Additionally, saturating the cornea with riboflavin creates a “shielding effect” in which the respective UV energy levels reaching the endothelium, lens and retina are titrated to a much lower intensity than the actual cellular damage thresholds. In fact, if a riboflavin-saturated cornea is at least 400µm in thickness, the UV irradiance transmitted to the endothelium is only 0.18mW/cm2, whereas the actual endothelial damage threshold is approximately 0.35mW/cm2. Thereafter, the energy level projected to reach the crystalline lens and retina is even lower compared with the respective damage thresholds of these tissue layers.14,15
3. What is the purpose of epithelial removal in the standard CXL protocol?
The lipophilic nature of the corneal epithelium and the small pore size of its tight junctions make this layer essentially impervious to riboflavin molecules. These epithelial barrier characteristics prevent efficient and homogenous riboflavin saturation in the targeted stromal tissue.16
Epithelium also contains enzymes with high antioxidant properties such as ascorbate and tryptophan residues, which can prevent UV penetrance and scavenge reactive oxygen species. Moreover, the presence of an epithelial barrier slows the rate of oxygen replenishment during CXL procedures, thus reducing the total amount of new cellular crosslinks that can be created. Consequently, when the same standard CXL protocol is carried out with an intact corneal surface, the procedure’s overall efficacy will be lower than anticipated. On the other hand, due to non-homogenous riboflavin saturation and reduced riboflavin shielding effects, UV transmissions delivered to the endothelium and deeper ocular tissues may be higher than previously calculated.16,17
Clinicians should not assume CXL is only effective when accompanied by epithelial debridement. Although transepithelial CXL (TE-CXL) applications do not currently have FDA approval, modified treatment techniques are under investigation to enhance TE-CXL efficacy.
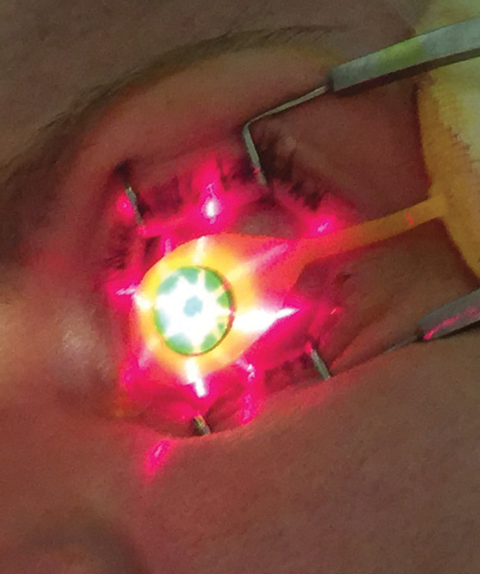 |
| Fig. 3. Crosshair guidance is projected from KXL device onto treatment site. |
4. How is the standard CXL protocol performed?
Topical anesthesia is used when removing the central 9mm of epithelium to ensure patient comfort and allow for faster, more homogenous stromal saturation of Photrexa viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) during CXL. This phase lasts for 30 minutes with riboflavin instillation in two-minute intervals.10
After 30 minutes, patients are examined under the slit lamp to ensure the riboflavin has saturated the intended treatment area and that it is present within the aqueous (Figures 2a and 2b). Per FDA approved indications, clinicians must perform pachymetry after riboflavin application to make sure the corneal thickness is at least 400µm. If it is less than 400µm, hypotonic Photrexa riboflavin should be administered every five to 10 seconds until the cornea is rehydrated to 400µm or greater.10
Once the appropriate pachymetry level is verified, clinicians use the KXL UV device (Avedro) for the second phase of CXL treatment, where 30 minutes of UV irradiance (3mW/cm2) yields a total energy dose of 5.4J/cm2.8 During the UV emission period, Photrexa viscous is instilled in two-minute intervals while proper centration and device-eye distance are maintained by the operator. The proper KXL device position can be guided by the crosshair image projections (Figure 3), which aid the delivery of an optimum illumination beam profile to the treated cornea.
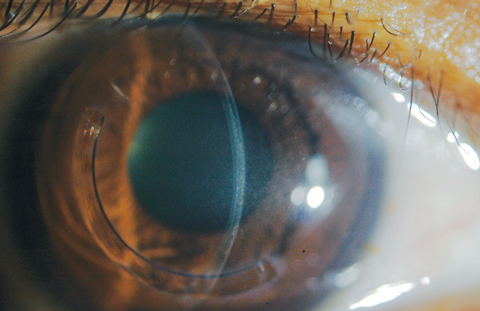
Excess riboflavin can be rinsed off with a balanced salt solution at the end of a treatment session. A bandage contact lens (BCL) is inserted after instillation of topical antibiotic and corticosteroid agents. The BCL should be kept on the treated eye for three to five days or until epithelial closure (Figures 4a and 4b).
 |
| Fig. 4a. Here, a bandage soft contact lens is on the eye immediately after CXL treatment on a patient where corneal riboflavin saturation is still evident. |
 |
| Fig. 4b. Epithelial wound closure is mostly complete on the same patient only three days after treatment with CXL. |
5. What are the patient selection recommendations?
In 2016, the standard CXL protocol received labeled indications in the United States to treat patients 14 years of age or older with progressive KCN or corneal ectasia following refractive surgeries. However, when left untreated, disease severity and rate of progression are known to be more aggressive in younger patients. Therefore, the KXL system and Photrexa/Photrexa viscous can be considered for off-label use in younger patients with minimum corneal thickness of 400µm or greater. KCN patients as young as eight have been reported by clinical trials, but special informed consents must be obtained from the patients and their guardians in these cases.18
Although the FDA has not specified any contraindications, clinicians should exercise judgment before offering CXL to lactating mothers and patients older than 65 years of age. Also, researchers strongly recommend avoiding CXL during the course of a pregnancy. A recent study found topographic, pachymetric and biomechanical evidence of KCN progression in 100% of its pregnant patient cohort.19 This led researchers to recommend discussing prophylactic CXL with female patients prior to family planning. Some European countries have begun to proactively offer CXL to female KCN patients who are planning for pregnancy despite lack of disease progression.20
6. Is KCN progression necessary to recommend CXL?
Although KCN progression is part of the on-label indication for CXL treatment, certain circumstances do not require progression before a CXL consult. Female KCN patients who are planning to become pregnant and patients at a high risk for progression are just two potential clinical examples.19,21
According to the conventional KCN care model, some amount of meaningful changes in clinical parameters must manifest prior to initiating a new treatment course. However, significant progression frequently occurs before action is taken due to the lack of consensus on the exact clinical indicator and corresponding magnitude of change that constitutes disease progression. Many CXL studies define KCN progression as changes over a 12-month period in the any of the following measurements: 1D or more in maximum keratometry; 0.5D or more in myopia; 1D or more in astigmatism; or 10µm or more loss in thinnest pachymetric point.10-12,20,22 However, with the limited accuracy of traditional topographers when imaging the irregular corneal surface and the refractive variability of KCN patients, these guidelines may result in a higher rate of false positives.
Alternatively, one expert panel recently recommended that the presences of at least two of three criteria can establish progression: steepening in anterior corneal curvature; steepening of posterior corneal curvature; or thinning when comparing pachymetric distribution profile from periphery to thinnest point.21 While useful, these guidelines require access to corneal tomography capable of tracking changes over time, presenting a possible challenge for some comanaging clinicians.
Given these clinical hurdles, the expert panel assembled from four supranational corneal societies concluded that CXL recommendations can be made to KCN patients with high-risk profiles, even if progression has not been documented.21
7. Should I consider CXL for patients older than 40?
The short answer is yes. KCN patients tend to display a slower rate of progression or even stabilization in their fourth or fifth decade of life—likely a byproduct of age-associated crosslinking. However, KCN expression is highly variable, and age alone is not always a well-defined end point for KCN. A retrospective chart review from Wills Eye Hospital found 24% of the 186 eyes newly diagnosed with KCN belonged to patients aged 40 or older.23
In addition, given that post-surgical ectasia can occur at a later point in life than a typical KCN patient, clinical consensus has not defined an age range for when ectasia typically occurs and when progression may slow down. Thus, clinicians should refrain from using age as an absolute contraindication for CXL candidacy.
8. What are the general CXL postoperative findings and expectations?
The initial phase of recovery from standard CXL is much like any procedure involving corneal epithelial removal. Although BCLs offer therapeutic protection and enhanced patient comfort, most patients still experience some ocular discomfort or pain until the epithelial defect closes, which usually occurs in three to five days.24
After epithelial closure, visual acuity generally worsens or greatly fluctuates throughout the first month before slowly returning to baseline by the third month. Patients may experience a mild improvement in vision between months three and six or months six and 12. Additionally, a stabilization trend typically emerges as the new baseline between months six and 12.10-12
After standard CXL, keratometry, pachymetry and transient CXL haze measurements also follow a similar temporal pattern, with further steepening, thinning and reduction in corneal transparency during the first month. These trends typically reverse over the following two months, at which point patients slowly return to baseline characteristics. Sometimes these patients even experience mild improvements before reaching a plateau of stabilization (Figure 5).10-12
It’s important to refrain from misconstruing these immediate post-operative trends as worsening in KCN disease or CXL failure. Overall, despite an epi-off CXL protocol, only a short period exists during the immediate postoperative recovery where patients may feel visually compromised. This is because patients are refit in contact lenses or can resume contact lens wear before they reach post-CXL stabilization.
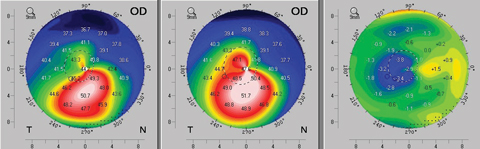 |
| Fig. 5. An example of topographic flattening seen as early as three months after standard (epi-off) corneal crosslinking protocol. The left map shows the patient’s pre-operative axial topography. The center map is the postoperative topography at month three, and the right map provides a difference calculation revealing the topographic improvement at month three. Click image to enlarge. |
9. Can CXL patients expect any refractive changes?
Studies have reported variable results for sphere, cylinder and spherical equivalent at 12 months post-CXL treatment. Some show statistically significant refractive changes, while others recorded no notable differences.25-27 Researchers have reported improvements in total higher-order aberration, spherical aberration and coma as well as average topographic flattening of 1.6D.10,28 Still, the literature provides no consistent correlations between changes in these clinical parameters and CXL treatment.
Consequently, KCN stabilization should remain the primary objective of currently available CXL protocols. Before recommending CXL, patients should be informed that contact lenses or glasses will still be required after CXL, and this management approach may improve patients’ quality of life by reducing the frustration often associated with frequent optical changes when KCN is left untreated.
10. Is CXL haze a concern?
Transient CXL haze can appear similar to post-PRK corneal haze. With experience, however, clinicians can differentiate the two entities under the slit lamp. CXL haze creates a dust-like tissue change in the anterior to mid-stromal levels, whereas PRK haze manifests in a reticulated fibrotic proliferation pattern that is localized to the subepithelial to anterior stromal layers. Given the different anatomic appearances and the self-resolving nature of CXL haze, it is unlikely to carry the same visual implications as PRK haze.24
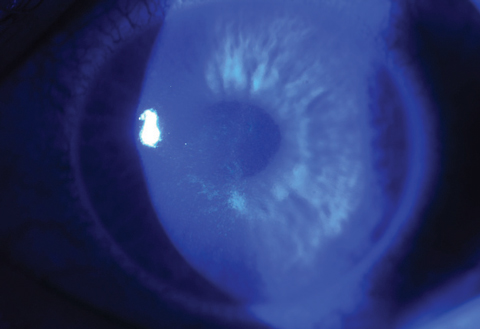
Immediately after CXL treatment, confocal microscopy will reveal keratocyte apoptosis and lacunar edema in the anterior to mid-stromal area. As areas of CXL haze and stromal edema start to show improvement by the end of the first month, clinicians will see zones of optical discontinuity—or demarcation lines—with an optic section during slit lamp examination (Figure 6).24
Although glare disability is a possibility during the first six to eight weeks, transient CXL haze and demarcation line depth are often used as indicators to reflect treatment penetration and resultant stromal collagen remodeling. As keratocytes slowly repopulate, the backscattering of light starts to resolve and the areas of CXL haze begin to fade between three and six months. The haze will often become unnoticeable by one-year post-CXL. Topical steroids are often discontinued after the first few weeks following the procedure, yet most cases of CXL haze self-resolve over time without further therapeutic interventions; thus, researchers suggest topical steroids do not mitigate CXL haze and their long-term use is not necessary after standard CXL. However, one study proposed that topical steroids may be justified if persistent haze or stromal scarring is observed after the one-year mark.24,29
 |
| Fig. 6. The demarcation lines are visualized with optic section in a patient who received off-label treatment of CXL and Intacs corneal implant (AJL Ophthalmic). |
11. Can you perform CXL without removing the epithelium?
Standard epi-off CXL is minimally invasive and highly effective in halting KCN progression. Additionally, adverse events are uncommon after standard CXL.10-13 However, researchers continue to investigate delivery methods to increase comfort during and after the procedure, shorten visual recovery time and reduce risks of potential infection.
Keeping the epithelium intact reduces diffusion rates of riboflavin, UV light and oxygen, all of which are essential to the photochemical reactions during CXL. Researchers have been able to bypass the epithelial barrier function by disrupting tight junctions with chemical enhancers such as benzalkonium chloride (BAK) and ethylenediaminetetraacetic acid (EDTA). These corneal enhancers are incorporated into the riboflavin solution to assist penetrance into corneal stroma. However, some studies have reported shallower demarcation lines and reduced corneal stiffening effects after TE-CXL.30,31
Although several studies reported higher regression rates with TE-CXL, its rates of adverse events are also lower than those of standard epi-off CXL. Additionally, the shallower CXL treatment depth may be advantageous in eyes with thinner corneas at baseline. Patients with a low risk of progression and those who are concerned about visual recovery time may be reasonable candidates for TE-CXL.24,30,31
Until the efficacy of TE-CXL improves, we will continue to recommend standard epi-off CXL for KCN patients with a high likelihood of progression or aggressive clinical progression.
12. When should I refit contact lenses after CXL?
A study using confocal microscopy showed that epithelial thickness gradually returns to normal between three and six months after standard CXL.24 However, many patients require contact lens rehabilitation to function and cannot wait six months before resuming contact lens wear.
Our personal approach is to adopt a lens fitting strategy that allows minimal to no interaction between the posterior lens surface and corneal epithelium, given the possibility of persistent haze with delayed epithelial healing or disrupted epithelial remodeling. Various lens designs can help accomplish this goal including those with corneal vaulting capacities, such as hybrid, scleral, piggyback and even custom soft lenses. From clinical experience, we have found the ideal time to consider refitting a lens is approximately four to six weeks after standard CXL or two weeks after TE-CXL. It’s also prudent to stress to patients, particularly after standard CXL, that frequent refractive modifications in their contact lenses may be expected over the next six to 12 months.
The emergence of CXL has ushered in a new era of KCN management in which clinicians no longer have to assume a passive reactive management approach and offer patients only a forced choice between contact lenses and corneal grafts. With early CXL intervention for appropriate candidates and continual post-CXL monitoring, clinicians can help patients maintain their best visual function and maximally defer the possible needs for keratoplasties. Today’s clinical focus should go beyond simply refitting contact lenses as KCN progresses. With early detection of KCN, access to CXL and advancements in specialty lens designs, clinicians can help their KCN patients live life to the fullest.
Dr. Chang is director of Cornea Specialty Lenses at Wills Eye Hospital–Cornea Service and director of clinical services at TLC Vision. He is an advisory board member for the International Keratoconus Academy, the Gas Permeable Lens Institute and the Optometric Cornea, Cataract and Refractive Society.
Dr. Rapuano is chief of Cornea Service at Wills Eye Hospital. He has published several books, numerous book chapters and over 175 peer-reviewed articles, including having co-authored The Wills Eye Manual.
1. Godefrooij DA, Gans R, Imhof SM, Wisse RP. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94(7):675-8. |

