 |
Any decrease in vision, with or without headache, can signal consequences that go beyond the eye. These consequences can be extreme and include such dire conditions as aneurysm or tumors or can stem from infections that have systemic implications, such as Lyme disease or syphilis. These patients always warrant an urgent comprehensive work up.
When an optic disc edema is identified, optometrists need to keep their differential diagnoses broad, and consider common and uncommon etiologies.
Any appropriate serologic or radiologic testing should be ordered swiftly, as these will help identify devastating conditions.
The Patient
A 14-year-old Hispanic female presented to the retina office regarding concerns of an edematous optic nerve, blurred vision of the left eye and headache. The blurred vision and headache were described as mild and constant since their onset 10 months prior. The patient’s medical history was positive for obesity and she denied using any medications. The patient had a positive family history of diabetes and hypertension.
During the patient’s initial visit, the entering visual acuities with correction were 20/25 OD and 20/40 OS (pinholed to 20/30 OS). Pupils were equal and reactive to light with no afferent pupillary defect. Extraocular muscles had smooth full range of motion with no pain and visual fields by confrontation were unremarkable. The patient’s preauricular nodes were normal and non-tender on palpation. Goldmann tonometry revealed IOPs of 16mm Hg in both eyes.
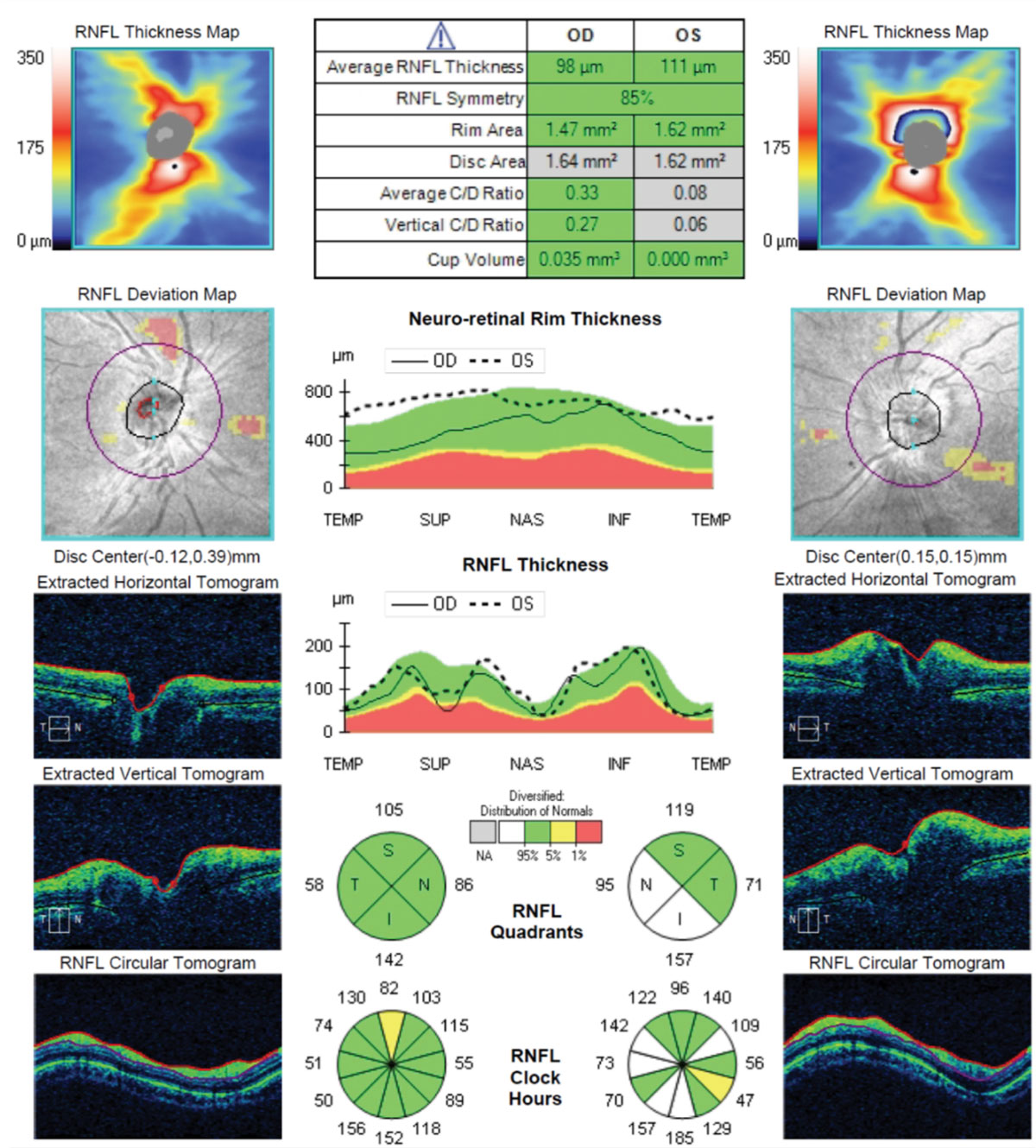 |
| Fig. 1. These SD-OCTs of the patient’s optic nerves and RNFL cube shows substantial elevation of her left eye’s neuroretinal rim tissue. The extracted tomograph highlights the large elevation asymmetry between the eyes. Click image to enlarge. |
Dilated fundus exam of the right eye was unremarkable; however, spectral domain optical coherence tomography (SD-OCT) revealed 2+ optic nerve swelling with blurred margins in the left eye (Figure 1). She also had an approximate cup-to-disc ratio of 0.2 OS, which was difficult to assess. No spontaneous venous pulsation was noted in either fundus. Macula, retinas, vessels and vitreous humor were unremarkable in either eye. Humphrey visual fields (HVF) 30-2 showed a few mildly incongruous depressed points within the superior temporal quadrants in the left eye more than the right (Figure 2). HVF reliability indices showed poor reliability on the left eye testing secondary to increased fixation losses.
We determined her left eye had an optic disc edema. The patient and parent were educated on the findings and the need for neuroimaging to be performed within one week. Blood work, magnetic resonance imaging (MRI) with and without gadolinium and lumbar puncture were ordered for further evaluation.
Follow-Up
At the next visit, the patient described her blurred vision as intermittent over the previous four weeks and denied any pain since the last exam. Visual acuity with correction was 20/25 OD and 20/40 OS, pin-holed to 20/25 OS. All entrance testing and anterior segment biomicroscope findings were unchanged since last exam. IOPs were 17mm Hg OD and 16mm Hg OS. Fundus findings were unchanged since previous visit. Fundus fluorescein angiography (FFA) and SD-OCT of the maculae were ordered and performed in office.
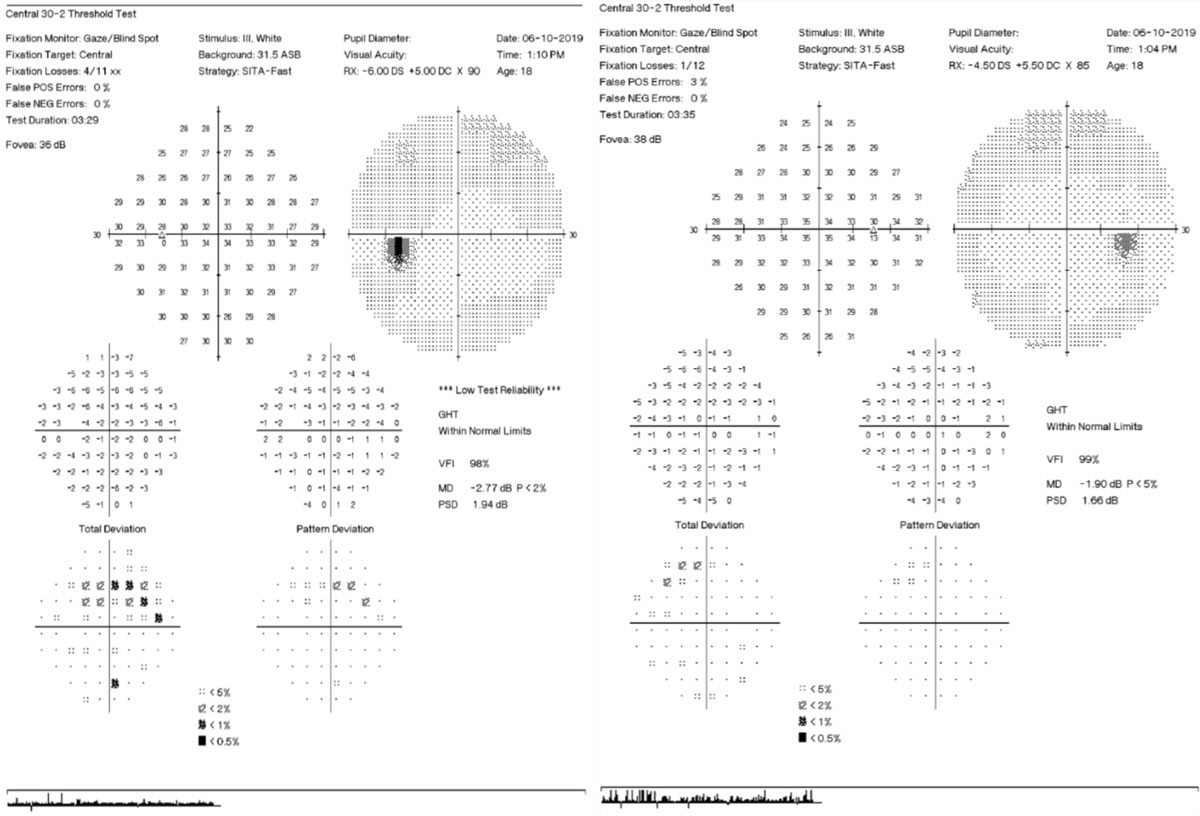 |
| Fig. 2. 30-2 visual field testing shows scattered superior arcuate defect points in both eyes, but more so in the left. Click image to enlarge. |
 |
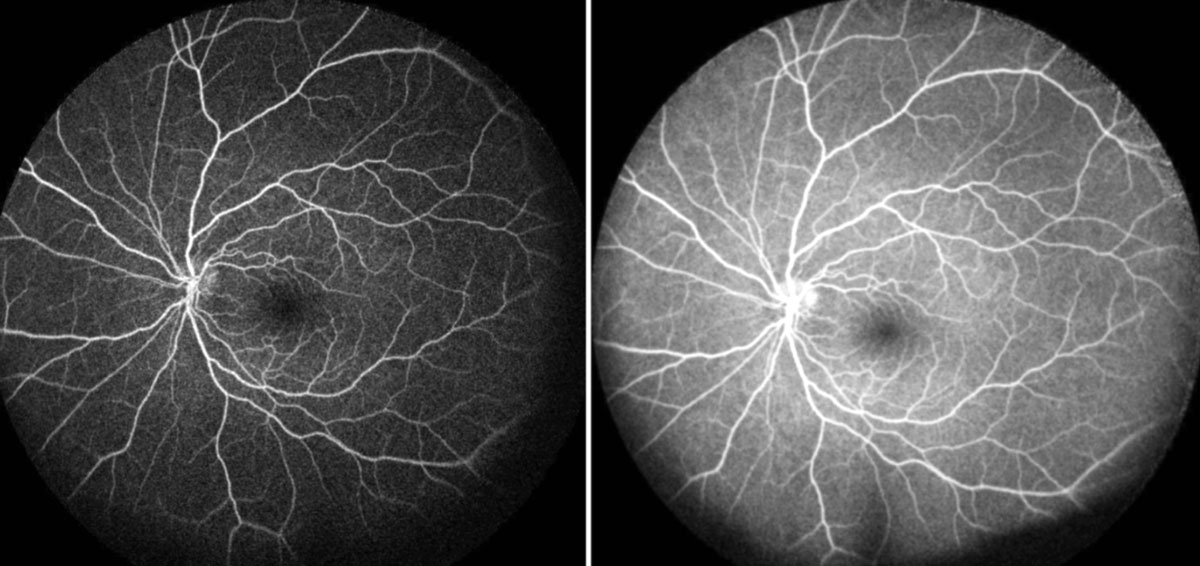 |
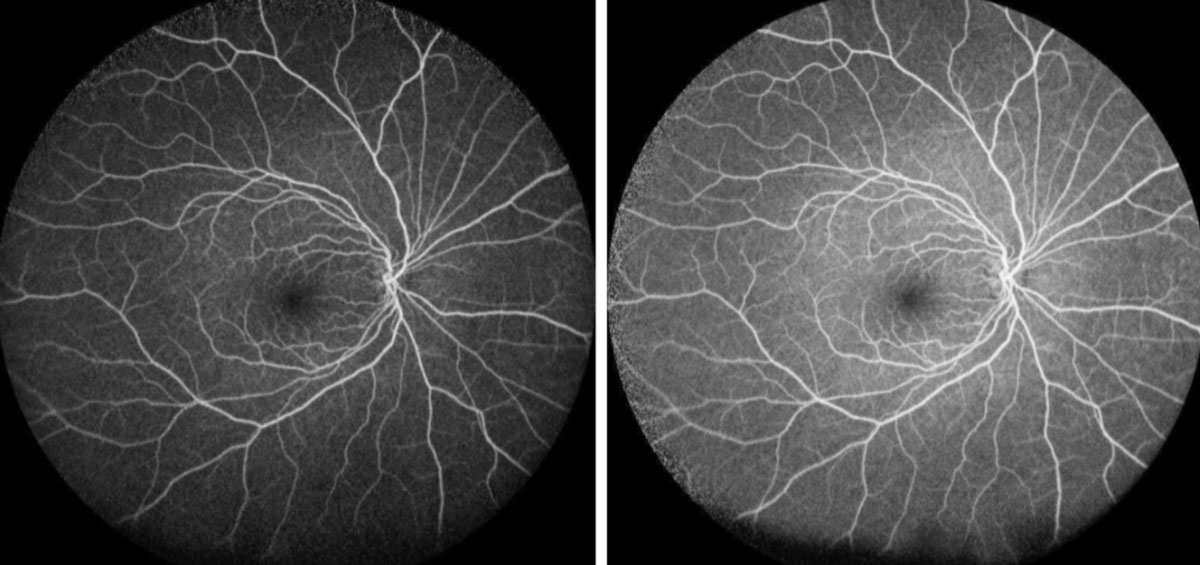
| Figs 3 and 4. Her right eye (top) is normal on FA, but her left eye (bottom) shows hyperfluorescent staining/pooling of the disc during the early and mid-phase. Click image to enlarge. |
FFA results were unremarkable in the right eye, but the left showed hyper-fluorescence of the superior disc during the arteriovenous phase (Figures 3 and 4). SD-OCT of the maculae showed no abnormal thickening or thinning and were generally symmetric between the right and left eyes (Figure 5). The results of the blood work revealed elevated rheumatoid arthritis factor and a slight elevation of circulating lymphocytes.
The MRI revealed a 1.4mm to 1.5mm nodular area of increased signal intensity projecting from the posterior left side of the anterior communicating artery, which is suspicious for a saccular aneurysm (Figure 6). Lumbar puncture was halted until further imaging, including a computed tomography angiography that could be performed to better delineate the aneurysm.
Differential Diagnoses
Unilateral disc edema is habitually bound with clinical suspicions of orbital compressive lesions, infection, inflammation and ischemia of the optic nerve.1-6 True unilateral papilledema is a rarity among all the potential clinical causes of optic disc swelling.2 As is the case with typical bilateral papilledema, unilateral papilledema occurs due to increased intracranial pressure.2
Compressive mass lesions of the optic nerves may arise in many different fashions, locations and sources. Rhabdomyosarcomas, optic nerve gliomas and optic sheath meningiomas are some of the neoplastic lesions to consider when dealing with the pediatric population. Rhabdomyosarcoma is the most common orbital malignancy in children and due to its sporadicity the cause is still unknown.7 Optic nerve gliomas and optic nerve sheath meningiomas can both cause gradual unilateral vision loss, relative afferent pupillary defect and unilateral proptosis.7,8
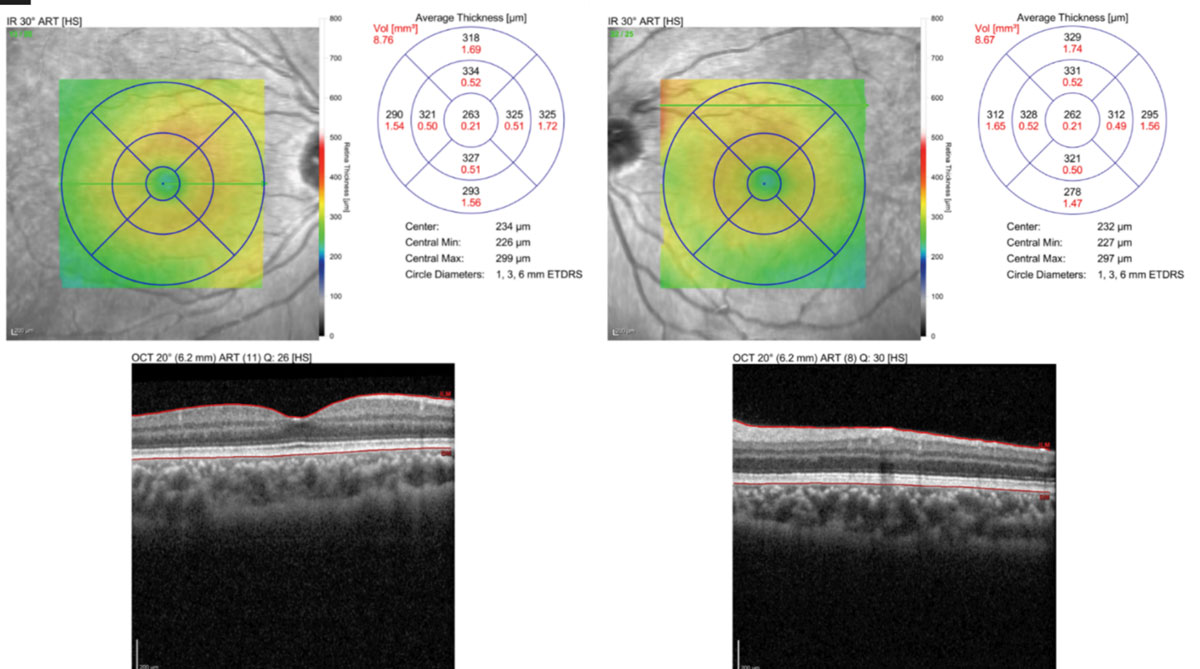 |
| Fig. 5. OCT macular scan shows no structural abnormalities. Click image to enlarge. |
Optic neuritis is an inflammatory morbidity that can manifest clinically as reduced visual acuity, with a painful swollen optic nerve.1,9 Optic neuritis has an array of etiologies but two that stand out for this case are multiple sclerosis (MS) and rheumatoid arthritis. MS should be considered as a differential when young patients complain of diminished vision accompanied by optic disc swelling. The use of MRI with and without gadolinium will help confirm a diagnosis of MS if white matter plaques are present. Optic neuritis may be associated with elevated serum rheumatoid factor secondary to systemic arthropathies.9
Anterior ischemic optic neuropathy (AION) has two varieties that can cause loss of vision.1,2 Both non-arteritic (NAION) and arteritic AION (AAION) manifest clinically as a loss of vision with a swollen nerve. NAION and AAION both typically affect patients over the age of 50 years old, the latter being extremely rare in patients younger than 50 years.1
Infectious optic neuropathy may be caused by Bartonella, syphilis, herpes, tuberculosis (TB) and Lyme disease, just to name a few.1,3 We ruled out these infectious causes with the appropriate serology including complete blood count with differential, QuantiFeron-TB Gold Plus test (Qiagen), Treponema pallidum antibody test and Lyme IgG/IgM test.
Diagnosis
This patient was ultimately diagnosed with left optic disc edema secondary to aneurysmal compression. Recent adult studies found that 35% of ruptured cerebral aneurysms were located at the anterior communicating artery.10 The true prevalence of unruptured aneurysms remains unknown.11 Patients with unruptured intracranial aneurysms may go on indefinitely with the diagnosis unbeknownst to them.
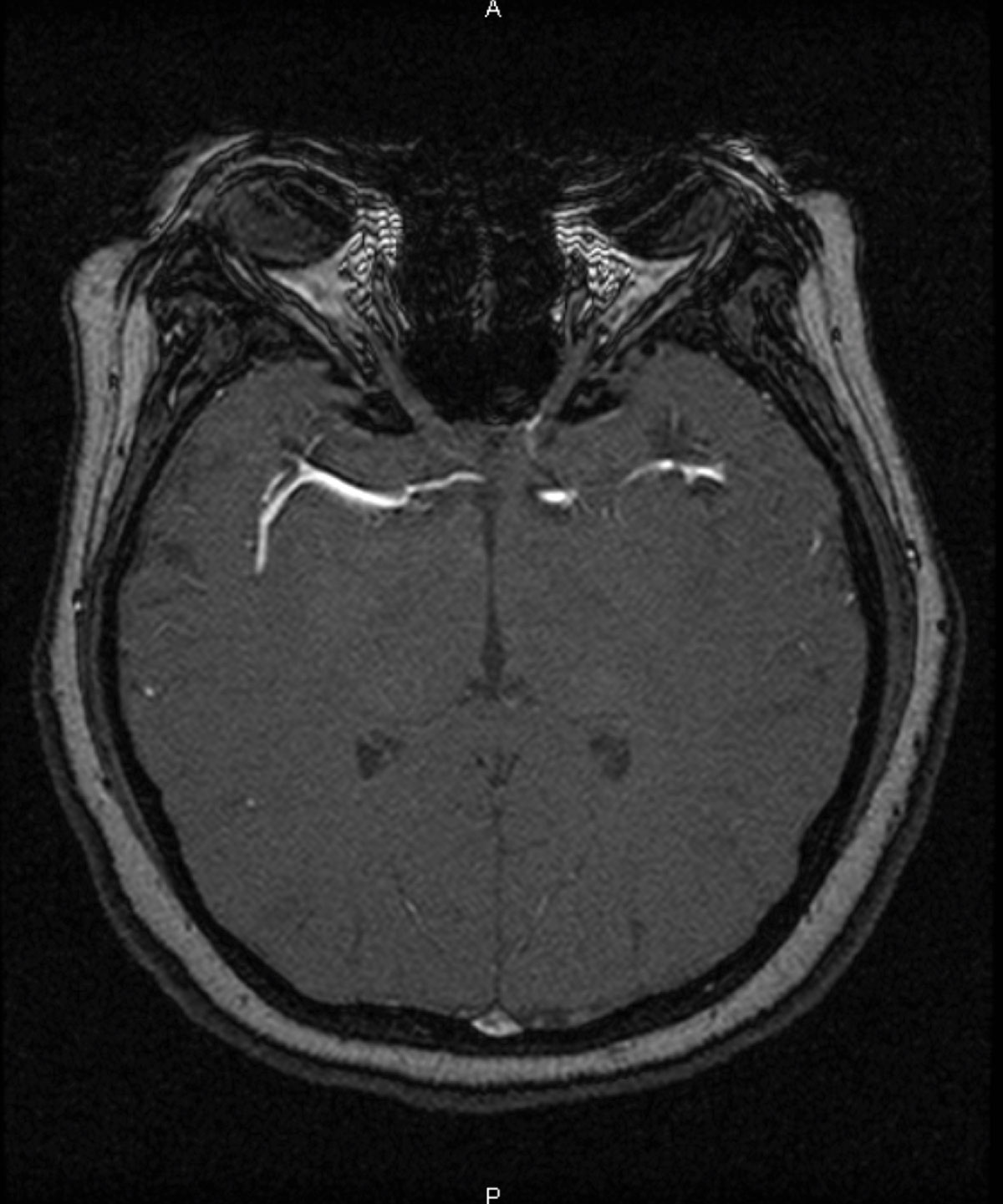 |
| Fig. 6. An MRI exposes a 1.4mm to 1.5mm saccular aneurysm of the anterior communicating artery. Click image to enlarge. |
The optic nerves are susceptible to aneurysmal compression due to their proximal anatomical location to the assembly of blood vessels that make up anterior portion of the circle of Willis. This compression of the optic nerve may be perceived by patients as blurred or diminished vision unilaterally or bilaterally.12
The discovery of an intracranial aneurysm in a pediatric patient is uncommon. Literature reports show only 0.5% to 4.6 % of aneurysms occur in patients 18 years or younger.13 In our case, the patient presented with signs and symptoms that may be associated with numerous etiologies. The anterior location of this patient’s aneurysm also speaks to the rarity of the case. The pediatric population appears to be more prone to intracranial aneurysms within the posterior circulation.13
Research shows only 5% to 10% of pediatric intracranial aneurysms occur at the anterior cerebral and anterior communicating artery.13
Our patient is currently under close monitoring by both our retina clinic and a local neuro-ophthalmologist for consideration of surgical options for the saccular aneurysm. Treatment for intracranial aneurysms vary based on the etiology and morphology. Management options include observation, endovascular coiling therapy, and surgical clipping with or without bypass surgery.13
The patient and parent were educated to follow up with their pediatrician regarding the elevated serum rheumatoid factor and to return to our clinic after surgical consult to be monitored for any retinal sequalae of the compressive lesion.
Dr. Mancha is a primary care resident at the University of the Incarnate Word.
Dr. Scales is a board certified ophthalmologist specializing in retina and uveitis as well as a clinical professor at the University of the Incarnate Word.
Dr. Pizzimenti is a full-time faculty member at the University of the Incarnate Word in San Antonio, Texas, where he coordinates the Primary Care Residency Program.
1. Kanski J, Bowling B. Kanski’s clinical ophthalmology: a systematic approach. 8th ed. Philadelphia:Elsevir;2015. 2. Hata M, Miyamoto K. Causes and prognosis of unilateral and bilateral optic disc swelling. Neuroophthalmol. 2017;41(4):187-91. 3. Kahloun R, Abroug N, Ksiaa I, et al. Infectious optic neuropathies: a clinical update. Eye and brain. 2015;7:59-81. 4. Kulkarni G, Singh RJ, Gadad V, et al. Unilateral papilledema in cerebral venous sinus thrombosis. J Neurosciences Rural Pract. 2017;8(Suppl 1):S106-10. 5. Shukla DP, Bhat DI, Devi BI. Anterior communicating artery aneurysm presenting with vision loss. J Neurosci Rural Pract. 2013;4(3):305-7. 6. Tufekci A, Kirbas S, Oner V, et al. Idiopathic intracranial hypertension presenting as unilateral papilledema: A case report. J Neurol Sci. 2013;333:e486. 7. Perry JD, Singh AD, eds. Clinical ophthalmic oncology orbital tumors. 2nd ed. Berlin, Heidelberg: Springer; 2014. 8. Shapey J, Danesh-Meyer H. Diagnosis and management of optic nerve glioma. J Clin Neurosci. 2011;18(12):1585-91. 9. Chen YH, Wang AG, Lin YC, Yen MY. Optic neuritis as the first manifestation of rheumatoid arthritis. J Neuroophthalmol. 2008;28(3):237-8. 10. Jou L, Lee D, Mawad M. Cross-flow at the anterior communicating artery and its implication in cerebral aneurysm formation. J Biomechanics. 2010;43(11):2189-95. 11. Turjman AS, Turjman F, Edelman E. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation. 2014;129(3):373-82. 12. Park J, Park S, Kim T, et al. Anterior communicating artery aneurysm related to visual symptoms. J Korean Neurosurg Soc. 2009;46(3):232-8. 13. Lv X, Jiang C, Li Y, et al. Endovascular treatment for pediatric intracranial aneurysms. Neuroradiology. 2009;51(11):749-54. |

