
Ever since the introduction of antibiotics in the 1940s, the very organisms that these medications were designed to destroy have been fighting back. In fact, four years after penicillin was introduced on the market, resistant strains of Staphylococcus aureus quickly emerged, as the bacteria developed a -lactamase enzyme, which rendered the antibiotic useless against resistant strains.1-3
In 1959, researchers developed methicillin as a narrow-spectrum, second-generation penicillin that was insensitive to the -lactamase enzyme. The unique chemical structure of methicillin made it effective against various strains of Staphylococcus aureus.
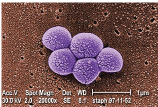
This colorized scanning electron micrograph depicts a grouping of methicillin-resistant Staphylococcus aureus bacteria (20,000x magnification). Courtesy: Jim Biddle and Janice Haney Carr
By 1961, however, a newer, stronger strain of Staphylococcus was discovered in the
In this article, well review antibiotic-resistant organisms, the systemic and ocular manifestations of Staphylococcus infections, and how to treat MRSA effectively.
Bactericidal Resistance
The evolution of resistant strains of bacteria is primarily the result of external selection pressure. External selection pressure refers to environmental conditions that enhance the emergence of resistant organisms. One of the most important external selection pressures is related to overuse of antibiotics, chiefly in medicine and agriculture.
One report estimated that 50 million pounds of antibiotics are taken in the United States every year.6 Approximately 33% of the antibiotics taken are for respiratory viral infections.6 When a patient does not appropriately use the antibiotic, or if he or she is treated with antibiotics for a non-bacterial infection, bacteria that harbors in the body may mutate and become resistant.
MRSA is one of these resistant bacteria. MRSA is very difficult to treat, and its prevalence is increasing at an alarming rate. During the past two decades, the incidence of MRSA infections in the
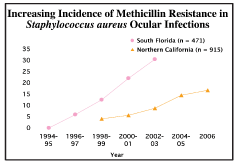
MRSA has become increasingly prevalent over the last decade in geographically diverse locations.12,13
HA-MRSA and CA-MRSA
Traditionally, most cases of MRSA have been acquired in a hospital setting (HA-MRSA). Risk factors for developing HA-MRSA include recent hospitalization or exposure to a health-care setting, residency in long-term care facilities, invasive or surgical procedures, intravenous drug use and prolonged exposure to antibiotics.8
More recently, however, cases of community-acquired MRSA (CA-MRSA) have surfaced in locker rooms, daycare facilities, public schools, playgrounds and stores. Worst of all, approximately 33% of all people carry CA-MRSA on their skin or in their nasal cavities without even knowing it.9-12 Even though most people who carry MRSA in this way do not develop an infection, the risk is always present. It has become a disturbing reality that these aggressive, difficult-to-treat bacteria strains are present in most communities. This change in epidemiology poses a significant threat to the successful treatment of common infections.
Perhaps surprisingly, CA-MRSA is not derived from HA-MRSA. In fact, CA-MRSA is distinctly different from HA-MRSAboth genetically and epidemiologically.8 Genetically, CA-MRSA isolates possess a shorter mecA gene than HA-MRSA isolates. This means that CA-MRSA isolates cannot encode for resistance to other antibiotic agents. As a result, CA-MRSA behaves more akin to methicillin-susceptible Staphylococcus aureus (MSSA), and is more vulnerable to a host of non--lactam antibiotics, whereas HA-MRSA tends to be resistant to a broad range of antimicrobial agents.8
These differences suggest that CA-MRSA represents dissemination of novel strains of MRSA within the community and is not an extension of hospital-acquired organisms into the community. Further, almost all strains of CA-MRSA are from a single clone (
From a disease state perspective, there also appear to be other differences between HA-MRSA and CA-MRSA. For example:
Diseases associated with HA-MRSA tend to occur in the bloodstream and/or respiratory and urinary tracts, while CA-MRSA strains are typically found on the skin and in soft tissues.
CA-MRSA more often occurs in younger individuals than HA-MRSA, and CA-MRSA is acquired in settings that involve crowding, constant contact and compromised hygiene.
Lesions linked to CA-MRSA often have necrotic centers that can be mistaken for spider bites.10
Unfortunately, it is becoming increasingly difficult to distinguish between these two unique strains of MRSA, as CA-MRSA is now being found in hospital settings. The primary danger is that the two MRSA strains might come into close proximity with one another, which could lead to additional antimicrobial resistance within all the strains of MRSA-related infections.10
Most recently, the Journal of the American Medical Association published a surveillance report of invasive MRSA from nine
MRSA and Ocular Health
Within eye care, there are increasing reports of MRSA-related infections. One study in northern California noted a 12.6% increase in ocular MRSA between July 1, 1998 and July 31, 2006.12 Fortunately, most cases were mild and non-sight threatening, with 78% manifesting as blepharoconjunctivitis, 4.6% as keratitis and 2.4% as endophthalmitis.14
The diagnoses associated with MRSA were not statistically different compared to other isolates. One-hundred percent of the isolates were sensitive to vancomycin, 93.2% were sensitive to tetracycline and 63.6% were sensitive to bacitracin. Ciprofloxacin and erythromycin were essentially ineffective, with sensitivities of 14.8% each.12 The authors concluded that the spectrum of ocular pathology caused by MRSA was milder than other reports suggest. That may not be the case in other geographically diverse locations.
In a study from south
Much like the study in northern
Refractive surgery is another important area of eye care that has to be on guard for the increase of MRSA. In a retrospective review of 13 cases of MRSA keratitis following refractive surgery, most infections were linked to a hospital setting.14 In this study, 12 of the 13 cases occurred in health-care workers or other individuals who were exposed to a hospital surgical setting. For infection prophylaxis immediately after refractive surgery, seven patients were prescribed a topical third-generation fluoroquinolone (ciprofloxacin 0.3% or ofloxacin 0.3%), one patient was prescribed tobramycin, one patient was prescribed erythromycin, and three patients were prescribed a topical fourth-generation fluoroquinolone (one patient had gatifloxacin 0.3%, one patient had moxifloxacin 0.5%, and the other patient had both medications). The prophylactic antibiotic for one patient was not known. Twelve of the 13 cases were treated with vancomycin (35mg/ml to 50mg/ml).
The authors concluded that MRSA keratitis is a serious and increasingly common complication following refractive surgery, and that patients who are exposed to a health-care environment should be considered at additional risk for MRSA keratitis.14 They also suggested performing scraping, culture and sensitivities of all cases of focal infiltrates after photorefractive keratectomy (PRK), lifting and scraping the flap in cases after LASIK, and eyelid cultures to determine if patients are carriers of the organism in order to prevent re-infection. Finally, the authors recommend preoperative treatment of blepharitis as well as the use of either a fourth-generation fluoroquinolone or bacitracin for topical prophylaxis.14
The fourth-generation fluoroquinolones are still preferred over previous generations because they inhibit both topoisomerase II and DNA gyrase, which require two separate mutations to develop resistance.15 On a molecule-to-molecule basis, the fourth-generation fluoroquinolones have not shown good efficacy against MRSA.15 However, when commercial preparations are compared, Zymar (gatifloxacin, Allergan) has a clear edge because of the presence of benzalkonium chloride (BAK).15
One study compared the minimum inhibitory concentration (MIC90) data of various strains of gram-positive bacteria with both the pure molecule and commercial preparation of gatifloxacin and moxifloxin.16 The MIC90 of the pure molecule was fairly equal between the two drugs, though moxifloxacin had a slight edge against various strains of Staphylococci and Streptococci organisms. When the commercial preparations were used, there was a much different outcome. Zymar demonstrated a two to 260 times lower MIC90 than that of Vigamox (moxifloxacin, Alcon Laboratories).15,16 This study also showed that Zymar was more effective against MRSA isolates than Vigamox.15,16
Another similar in-vitro study compared the addition of BAK to various concentrations of gatifloxacin and moxifloxacin.17 It showed that, regardless of the antibiotic used, the addition of 0.005% BAK to either fourth-generation fluoroquinolone significantly reduced bactericidal time with various Staphylococci isolates from four hours to less than one hour. These results demonstrate that the presence of BAK can have an additive effect.17
Vancomycin is regarded as the gold standard and first treatment of choice for a MRSA infection. Other drugs that have shown excellent susceptibility against ocular infections related to MRSA include bacitracin and Polytrim (polymyxin B/trimethoprim, Allergan).
For patients who have post-LASIK infections, irrigate under the flap with fortified vancomycin. Topical therapy should include alternating between fortified vancomycin every 30 minutes and a fourth-generation fluoroquinolone, as well as bacitracin ointment q.i.d. to the lids. However, do not use steroids. Take extra precautions, such as preoperative cultures and treatment, for patients who are at risk for MRSA colonization.14
To manage a corneal ulcer or other ocular infections, consider two crucial precautions to prevent evolution of resistance. First, never taper an antibiotic. A short-term, high dosing schedule should barrage the inciting bacterial agent and leave no survivors. Lower dosing merely extends the time the organism will have to complete the life cycle and possible mutate to develop resistance.
Secondly, if unsure of the offending agent at the time of presentation, avoid shotgun therapy and consider other possible etiologies, such as viral or fungal infections. With the rise of MRSA, culturing is even more important than in the past so that appropriate therapy can be administered.
Meanwhile, there are new treatments in the pipeline. Research is currently under way on the potential use of Zyvox (linezolid, Pfizer) for prophylaxis and treatment of MRSA keratitis.18,19 Zyvox, a synthetic antibiotic in the oxazolidinone drug class, disrupts the initiation process in protein synthesis earlier than other drugs that work by similar mechanism, including chloramphenicol, clindamycin, the aminoglycosides and the macrolides. It is bacteriostatic against Enterococci and Staphylococci, and bactericidal for Streptococci. Zyvox is highly absorbed, may be taken orally, and reportedly has a bioavailability of 100%.20 It has been FDA-approved for several drug-resistant strains of Enterococcus, Staphylococcus and Pneumococcus.
This patient demonstrated choroidal neovascularization, which was treated with Avastin (bevacizumab, Genentech). Following the Avastin injection, she developed MRSA endophthalmitis.
A Note on Prevention
To prevent the spread of MRSA infection, simple hygiene is very effective. Meticulous hand washing and sensible personal hygiene considerations are at the core of any prevention plan. Additionally, cover skin infections, wear latex gloves, and dispose of dirty or soiled wraps and bandages. Alcohol and chlorine bleach have been proven to be effective against MRSA, and routine cleaning can help prevent nosocomial infections.20
The rise in multiple drug-resistant strains of MRSA in otherwise healthy individuals has concerned both the public and the medical community. The transmission of MRSA from hospital settings heightens the need for prevention and appropriate antimicrobial treatment in both the systemic and ocular arenas.
Clearly, there is a need for newer and stronger antibiotics that can fight infection and be resilient to developing bactericidal resistance. At the same time, doctors need to be careful about overprescribing antibiotics. Other measures, such as improving infection control, will go a long way toward preventing and fighting infection. In the meantime, clinicians should use the strongest and most effective drugs that are currently availablefourth-generation fluoroquinolones. Use of these agents provides the best chance to effectively kill and destroy all the bacteria that are present so resistance does not ever have the chance to develop.
Dr. Dunbar is director of optometric services and optometry residency supervisor at Bascom Palmer Eye Institute in
1.Chambers HF, Sande MA. Antimicrobial Agents: General Considerations. In: Hardman JG, Limbird E (eds). Goodman and Gilman"s the Pharmacological Basis of Therapeutics. 9th ed.
2. Lewis R. The Rise of Antibiotic-resistant Infections.
3. Totar K. Bacterial Resistance to Antibiotics, Todar"s Online Textbook of Bacteriology. Available at: http://textbookofbacteriology.net/resantimicrobial.html (Accessed November 7, 2008).
4. Jevons MP. Celbenin-resistant Staphylococci. BMJ 1961; 1:124-5.
5. Chang S, Sievert DM, Hageman JC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene.
6. Sensakovic JW, Smith LG. Oral antibiotic treatment of infectious diseases. Med Clin North Am 2001 Jan;85(1):115-23.
7. Hawkes CA. Antibiotic resistance: a clinician"s perspective. Mil Med 2000 Jul; 165(7 Suppl 2):43-5.
8. File TM Jr. Impact of community-acquired methicillin-resistant Staphylococcus aureus in the hospital setting. Cleve Clin J Med 2007 Aug;74 Suppl4:S6-11.
9.
10. Kuehnert MJ, Kruszon-Moran D, Hill HA, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J Infect Dis 2006 Jan 15;193(2):172-9.
11. KIevens, RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007 Oct;298(15): 1763-71.
12. Freidlin J, Acharya N, Lietman TM, et al. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am J Ophthahnol 2007 Aug;144(2):3135.
13. Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115(1):51-56.
14. Solomon R, Donnenfeld ED, Perry HD, et al. Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. Am J OphthalmoI 2007 Apr; 143(4):629-34.
15. Callegan MC, Novosad B. Efficacy of fourth-generation fluoroquinolones against gram-positive species commonly involved in ocular infections. Presented at: Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); April 30-May 4, 2006;
16. Snyder R, Callegan MC, Novosad B. Killing of methicillin-resistant S. aureus (MRSA) and S. epidermidis (MRSE) ocular isolates by fourth-generation fluoroquinolone ophthalmic solutions. Presented at: American Society for Refractive and Cataract Surgery Symposium (ASCRS); April 27-May 2, 2007;
17. Kowalski RP, Kowalski BR, Romanowski EG, et al. The in vitro impact of moxifloxacin and gatifloxacin concentration (0.5% vs. 0.3%) and the addition of BAK on antibacterial efficacy. Am J Ophthalmol 2006 Nov; 142(5):730-5.
18. Weigelt J, Kaafarani HM, Itani KM, Swanson RN. Linezolid eradicates MRSA better than vancomycin from surgical-site infections. Am J Surg 2004 Dec;188(6):760-6.
19. Ament P, Jamshed N, Horne J. Linezolid: its role in the treatment of gram-positive, drug-resistant bacterial infections. Am Fam Physician 2002 Feb 15;65(4):663-70.
20. Methicillin-resistant Staphylococcus aureus (MRSA).

