 |
Q:
I just diagnosed early open angle glaucoma in a 34-year-old patient who is six months pregnant. What are my options on drops, and does anything change after she has the baby and begins nursing?A:
With 6.3 million pregnancies reported in the U.S. each year, doctors face the prospect of tailoring therapy to both mother and baby during an especially vulnerable time, says Caroline Pate, OD, Associate Professor at University of Alabama School of Optometry.1
Address potential concerns early and let patients know which ocular changes may be in store, she says. Among these changes: a natural reduction in IOP. “It’s rare to make a diagnosis of glaucoma during pregnancy, because of a natural decrease in intraocular pressure (IOP),” says Dr. Pate. She explains that the increased uveoscleral outflow pathway and decreased episcleral venous pressure, thought to be governed hormonally, typically results in a 19.6% IOP reduction in healthy patients and a 24.4% decrease in ocular hypertensives.2 “We often set a target IOP 20% to 30% lower than baseline when initiating glaucoma treatment. Pregnant patients’ IOP may actually drop this amount without therapy!” This may persist several months postpartum.
Risk vs. Reward
Though a need for IOP reduction is rare in these patients, carefully consider the benefits and risks of drugs in the pregnant patient, she advises. The FDA’s risk categories, though recently abandoned, can still help. “Medications in Category A or B are generally accepted safe to use during pregnancy, whereas Category C are prescribed only when the benefit justifies potential risks to the patient and baby,” says Dr. Pate. “Categories D and X are unsafe during pregnancy.”
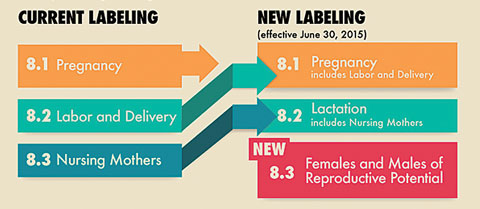 |
| New FDA labeling is more sensitive to risk profiles but puts the onus on ODs. |
Drugs approved after June 30, 2015, no longer use this classification system. “Doctors must now read the package inserts and analyze the safety data to make an informed decision,” she says. Drugs approved on or after June 30, 2001 will be phased-in, Dr. Pate explains.
Since no new topical agents have been approved since the new labeling system was initiated, “we can still refer to the more familiar pregnancy category labeling,” says Dr. Pate. Alphagan (brimonidine, Allergan) is the only available drop that falls into the Category B, she explains. “Generally considered safe during pregnancy, avoid Alphagan during lactation since it’s been linked to CNS depression and sleep apnea in breastfeeding infants.”3,4
Oral prostaglandins are sometimes used to induce labor.5 Though it’s not proven that ocular topical prostaglandins result in a similar effect, it is probably wise to avoid them, she notes. Topical β-blockers should be also be avoided, due to the risk of fetal cardiac arrhythmias.6 Oral carbonic anhydrase inhibitors given during pregnancy have been linked to congenital malformations, so it’s best to avoid the topical counterparts as well, Dr. Pate explains.7
What is considered safe during pregnancy may not be safe during lactation and vice-versa, says
Dr. Pate, and she recommends a free, peer-reviewed online database of from the US National Library of Medicine. “The LactMed database includes helpful information such as levels of a particular drug in breast milk, infant levels in blood, potential effects in breastfeeding infants and on lactation itself. Useful apps also exist for ease of use.”8
Though topical IOP-lowering drugs generally pose little risk to the fetus, one must still consider the risks and benefits when prescribing, says Dr. Pate. “Treating these glaucoma patients can be challenging. If in doubt, consult the patient’s OB/gyn or PCP prior to treatment.”
|
1. CDC. Pregnancy Rates for U.S. Women Continue to Drop. CDC Website. www.cdc.gov/nchs/data/databriefs/db136.htm. Accessed 9/15/16. 2. Omoti AE, Waziri-Erameh JM, Okeigbemen VW. A review of the changes in the ophthalmic and visual system during pregnancy. Afr J Reprod Health. 2008 Dec;12(3):185-96. 3. Trad MJ. Some ophthalmic drugs not safe for use in lactating or pregnant women. Helio. Primary Care Optometry News. 2008 September. 4. Autry J. Pregnancy precautions: how to prescribe safely for new and expectant mothers. Review of Optometry. 2016 Jan;153(1):28-33. 5. O’Brien WF. The role of prostaglandins in labor and delivery. Clin Perinatol. 1995 Dec;22(4):973-84. 6. Wagenvoort AM, van Vugt JM, Sobotka M, van Geijn HP. Topical timolol therapy in pregnancy: is it safe for the foetus? Teratol. 1998:58:258-62. 7. Al-Saleem AL, Al-Jobair AM. Possible association between acetazolamide administration during pregnancy and multiple congenital malformations. Drug Des Devel Ther. 2016 Apr 15;10:1471-6. 8. National Institutes of Health. LactMed Database. NIH. toxnet.nlm.nih.gov/newtoxnet/lactmed. Accessed 9/15/2016. |

