The art of practicing optometry is a combination of understanding disease states and current treatment protocols, educating patients to maximize compliance efforts, and understanding when to involve other practitioners into the care of your patient. You may recruit another practitioner for a second opinion on a patient’s condition or for consideration of surgical treatment of their condition.
It is this critical component that we will discuss in this article as it pertains to the management of glaucoma. Indeed, patient care ultimately is enhanced when eye care professionals, primary-care optometric physicians and tertiary-care subspecialists cooperatively and respectfully combine their expertise in facilitating the perioperative care of glaucoma patients. Besides thorough and efficient communication between providers, optometrists must understand the surgical processes, and master the associated preoperative and postoperative care components to not only maintain continuity of care but also to prevent unnecessary vision loss from the serious disease of glaucoma.
So, as a private practitioner, ask yourself these four questions before referring a patient with glaucoma for further evaluation.
1. Do I Have the Equipment to Diagnose and Manage the Disease?
Diagnostic technology for glaucoma is evolving at a rapid pace. Necessary diagnostic tests for your patient with glaucoma may include:
• Imaging instrument. Currently there are three forms of imaging technology that will aid in the early detection and management of glaucoma: scanning laser polarimetry (GDx), confocal laser scanning tomography (HRT), and optical coherence tomography (OCT).
These instruments can provide critical insights into early structural changes that may precede functional changes or physical changes detected by a dilated examination.1 Imaging technology allows the clinician to determine whether or not the patient’s optic nerve head or nerve fiber layer thickness is in the normal range.
Although this is an important consideration, it may be just as important, if not more important, to compare readings over time, paying particular attention to changes that may be occurring.2
The benefits that these technologies deliver to glaucoma management make it critical that you have access to one of them if you are managing this disease. If you do not own this technology, you should determine if you could have access to it for your patients. A referral to a regional center to perform the test may be an option.
But don’t overlook other possible options that may exist. For example, do you know an optometric colleague who may offer the technology to your glaucoma suspects and patients? Would sharing the cost of portable technology with a number of offices, and rotating it among the offices, be an option to offset some of the cost of buying it? If you have multiple locations, would having the technology in a central office and referring to that location for measurements be an option? They are all possibilities to feasibly offer the technology to your patients. Determine which one is best for your practice situation and make it a part of your glaucoma protocol.
• Optic nerve photos/perimetry. The same philosophy could be used for both optic nerve head photography and visual field analysis. These are technologies that should be utilized to monitor glaucoma suspects and for glaucoma management. If you do not have access to either of the technologies in your office, consider the options described above.
• Pachymetry. Measuring corneal thickness has also become the standard of care for glaucoma suspects as well as those diagnosed with the disease. It is well established that ocular hypertensive patients with thinner central corneal thickness are more likely to develop glaucoma than those patients with thicker corneas.3
Additionally, several studies have demonstrated that those patients with thinner corneas are more likely to advance in their severity of disease.4,5 Pachymetry instruments are not highly expensive, so every office that manages glaucoma should have one available.
• Gonioscopy. Additionally, gonioscopy should not be forgotten as a regularly-performed test on glaucoma suspects or those who have been diagnosed with the disease. Gonioscopy is typically performed once a year for those who are suspects or have glaucoma. A variety of gonioscopy lenses are available. (As a personal preference, a four-mirror lens is easily placed on most patients’ eyes, even if the patient may be slightly apprehensive or if they have a small interpalpebral fissure. Additionally, all four quadrants can be examined without having to rotate the lens.)
2. Am I Comfortable Managing this Patient?
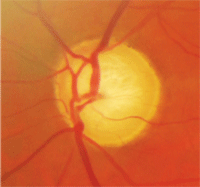
Patients who meet their IOP targets but continue to progress may require a
surgical consult. Courtesy: Edward Chu, O.D., and Ania Hamp, O.D.
This will depend in part on your experience in working with glaucoma patients and glaucoma suspects. We are fortunate in that we each practice in a multi-doctor settings that offer the ability to easily consult with other doctors in the practice for a second and, when needed, third opinion on challenging cases.
If you do not have another optometrist in your own practice, it may be wise to develop a relationship with an optometric colleague who you feel comfortable discussing challenging cases with. Additionally, having the patient be seen for a second opinion by colleague who emphasizes glaucoma care is also a viable option. Of course, a regional ophthalmologic referral center may be preferred if you feel that surgical intervention may be warranted.
3. Is the Patient Using Too Many Medications?
We are fortunate that we have a number of glaucoma medications that can be used remarkably successfully as first-line therapy for many of our ocular hypertensive and glaucoma patients. Typically, first-line therapy involves utilizing prostaglandin analogues. There are three medications in this class: Lumigan (bimatoprost 0.01% or 0.03%, Allergan), Xalatan (latanoprost 0.005%, Pfizer) and Travatan Z (travoprost 0.004%, Alcon). Each medication in this category is indicated for a once-daily dosing. (Travatan Z has the added advantage of being preserved with SofZia, an alternative to benzalkonium chloride.)
Typically the second class of medications includes either a beta blocker, an alpha adrenergic agonist, or a carbonic anhydrase inhibitor. These medications have an additive effect to the prostaglandin analogues because they work at different receptors in the eye.6
At times a third medication is needed. We are fortunate that we have the option of combination agents such as Combigan (brimonidine/timolol, Allergan), which is an alpha adrenergic agonist and a beta blocker, and Cosopt (dorzolamide/timolol, Merck), which is a carbonic anhydrase inhibitor and a beta blocker. These combinations offer the convenience of fewer drops a day for the patient.
Additionally, these combinations offer less BAK exposure to the ocular surface. This becomes a significant issue when a third medication is needed. Studies have shown an increase in conjunctival inflammatory cells and reduction in tear function for people using BAK containing products.7-9
When a third bottle is required to control intraocular pressures, consider surgical consultation. Depending on the patient, some will prefer to continue with their medical therapy while others would prefer a surgical consultation.
In addition to decreasing the BAK load to the ocular surface, we also need to take compliance into account. There is always the concern of non-compliance with any treatment regimen, but as the number of medications increase, so do our concerns that non-compliance may be an issue.10 As the number of medications increase, take into account both the ocular surface concerns and patient compliance, and consider surgical alternatives for lowering intraocular pressure.
Patients who meet their IOP targets at their office visits but continue to progress may be using their medications only prior to their visit and not using them in the interim, causing glaucomatous progression to occur. This will confuse the clinical picture, because we may assume good compliance and good IOP control.
When noncompliance is considered to be contributing to glaucoma progression, surgical consultation should be considered to minimize the effects of noncompliance.
4. Is the Patient Progressing Despite Achieving Target IOP?
It is a difficult situation when the goals that we have set for intraocular pressure have been reached and yet progression still occurs. In this instance, new intraocular pressure goals may be set that are lower than the original ones.
But if those goals cannot be met with medication, or if the medicines have significant side effects, or if the patient is noncompliant in using them, then a surgical consult is certainly warranted. If this is the case, selective laser trabeculoplasty (SLT) or filtering surgery may be indicated, contingent on the consultation of a highly skilled glaucoma surgical specialist. Fortunately, medicines alone, or in combination with laser treatment, effectively control IOP in a majority of patients.
While this is the classic approach, patients today often undergo surgery earlier in the course of the disease. Modern evidence suggests earlier control of IOP improves the chances of good long-term vision and a patient’s overall quality of life.11
Surgical approaches for managing POAG include filtering surgery (trabeculectomy), tubes/valves/shunts, and cyclodestructive procedures.12 Each method has advantages and disadvantages, and no single procedure is ideal for all situations. (See sidebar, “What Happens in Trabeculectomy”)
In short, it’s usually better to consider a progressing patient for surgery earlier, particularly in younger patients who do not want to risk a compromised quality of life through loss of vision.
What Happens in Trabeculectomy
Filtering surgery is the most effective surgical method to lower IOP,
and trabeculectomy is the most common filtering procedure. As such,
let’s discuss it step-by-step.
• Day of surgery. In trabeculectomy, the
surgeon creates a drainage channel that allows aqueous to leave the eye
more easily, thus lowering IOP. The average IOP drop after filtering
surgery is significantly greater than the reduction associated with
either medicines or laser treatment. Surgery by accomplished surgeons is
successful at least 90% of the time.13 The concurrent usage
of anti-metabolites, such as mitomycin C, increases success by
preventing postoperative scarring and bleb failure.14
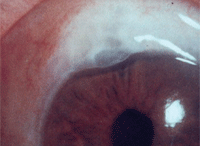
Trabeculectomy with mitomycin C. Notice the diffuse, moderately elevated, cystic bleb.
Trabeculectomy takes approximately 20
minutes to perform and is essentially painless, although some mild
discomfort and irritation is expected for a few days to few weeks
afterwards.
A subconjunctival steroid injection, a
combination antibiotic/steroid ointment, and a cycloplegic drop are
administered at the time of surgery. The eye is patched for one day, and
patients should wear an eye shield at bedtime for up to two weeks
following the procedure.
• Day one. Postoperative care at day one
involves carefully evaluating the IOP, anterior chamber depth, and the
nature of the filtering bleb. The ideal postoperative situation at day
one for trabeculectomy patients is low IOP (0mm Hg to 8mm Hg), a deep
anterior chamber and a good functioning bleb. Any significant variations
of these three critical findings need to be clinically addressed and
managed appropriately in a timely manner to avoid complications.
Therefore, such immediate postoperative care typically is rendered at
either the glaucoma surgeon’s office or a comanagement center where
glaucoma perioperative care may be rendered in conjunction with an
associated glaucoma surgeon.
If the first day shows findings within
normal limits, postoperative medications are initiated, including a
topical fluoroquinolone q2h, a topical corticosteroid (such as 1%
prednisolone acetate) q2h, and a cycloplegic (such as 1% atropine) q12h.
If all clinical findings appear within normal limits, there are
virtually no limitations on activities, with the exception of having
patients avoid heavy physical labor and unclean environments for two
weeks.
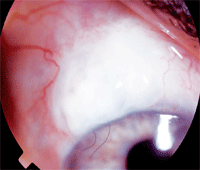
Trabeculectomy with mitomycin C. Notice the fairly localized, moderately elevated bleb with two releasable sutures.
• Week one to three months. The eye is
observed again at day two or three, and at one week. Within two weeks,
the suture(s) at the partial-thickness scleral flap may be lysed or
pulled if further IOP reduction is required, although up to four weeks
if an anti-metabolite was used.15 Careful in-office and
proper at-home ocular massage may be initiated after all sutures have
been removed to help maintain bleb function by forcing aqueous through
the drain and preventing from closing or scarring down. This may be
indicated for a few weeks.
The patient’s IOP, bleb and anterior
chamber should be carefully reevaluated on a weekly basis until week
six. Postoperative medications may be discontinued by three months if
treatment is proceeding well. The antibiotic and cycloplegic are
generally discontinued after one week, but the corticosteroid drop is
slowly tapered over eight to twelve weeks.
Postoperative complications associated
with glaucoma filtering surgery may include hemorrhage and hyphema,
earlier cataract formation, an encapsulated bleb, dellen formation and
infection.16 Infection of the bleb is rare, but is always the
chief concern. It also may occur during the late postoperative period
and can potentially lead to endophthalmitis.
All glaucoma patients require a lifetime of care and careful monitoring of their optic nerves, visual fields and intraocular pressures. If the patient has been diagnosed with normotensive glaucoma, more aggressive treatment may be required.
By contrast, if the patient has minimal optic nerve damage, the regimen should likely be less aggressive. In all glaucoma cases, whether or not medical or surgical treatment has been implemented, a target IOP should be determined and adjusted as indicated and hopefully achieved.
As optometric physicians continue to enhance their clinical skills to benefit glaucoma patient care, they should be encouraged to develop sound interprofessional relationships and guidelines for comanaging not only the normal postoperative course, but also recognizing and comanaging complicated postoperative outcomes.
Optometric physicians have a key responsibility to prevent blindness from glaucoma through meticulous diagnostic and treatment measures and interprofessional referral patterns that benefit the overall care of the patient. Work closely with either your comanagement center or directly with your glaucoma specialist for optimal care.
Dr. Brujic is a partner of Premier Vision Group, a four-location optometric practice in northwest Ohio, and a monthly columnist for Review of Cornea & Contact Lenses.
Dr. Pohl is clinical director at Pacific Cataract & Laser Institute, in Bellevue, Wash., an optometric consultation and ambulatory surgical center specializing in medical and surgical eye care. Dr. Pohl is a frequent lecturer and author on the topic of ocular disease comanagement, and is a founding Fellow of the Optometric Retina Society.
1. Xin D, Greenstein VC, Ritch R, et al. A comparison of functional and structural measures for identifying progression of glaucoma. Invest Ophthalmol Vis Sci. 2011 Jan 25;52(1):519-26.
2. Townsend KA, Wollstein G, Schuman JS. Imaging of the retinal nerve fibre layer for glaucoma. Br J Ophthalmol. 2009 Feb;93(2):139-43.
3. Manni G, Oddone F, Parisi V, et al. Intraocular pressure and central corneal thickness. Prog Brain Res. 2008;173:25-30.
4. Herndon LW, Weizer JS, Stinnett SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004 Jan;122(1):17-21.
5. Kim JW, Chen PP. Central corneal pachymetry and visual field progression in patients with open-angle glaucoma. Ophthalmology. 2004 Nov;111(11):2126-32.
6. Bournias TE, Lai J. Brimonidine tartrate 0.15%, dorzolamide hydrochloride 2%, and brinzolamide 1% compared as adjunctive therapy to prostaglandin analogs. Ophthalmology. 2009 Sep;116(9):1719-24.
7. Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994 Nov;112(11):1437-45.
8. Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998 Jan;82(1):39-42.
9. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999 Mar;106(3):556-63.
10. Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005 May;112(5):863-8.
11. Law SK, Nguyen AM, Coleman AL, Caprioli J. Severe loss of central vision in patients with advanced glaucoma undergoing trabeculectomy. Arch Ophthalmol 2007 Aug;125(8):1044-50.
12. Ayyala RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol 2000 Sep-Oct;45(2):91-105.
13. Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004399.
14. Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005 Oct 19;(4):CD002897.
15. Aykan U, Bilge AH, Akin T, etal. Laser suture lysis or releasable sutures after trabeculectomy. J Glaucoma. 2007 Mar;16(2):240-5.
16. Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005 Jul;140(1):16-22.

