

To determine why dry eye therapy fails, we must first define what we mean by failure. For example, is it failure if a patient who has been diligent with his treatment regimen and whose objective clinical findings suggest improvement remains symptomatic?
Or, is it failure if a patient has significant lissamine green staining, rapid tear break-up times and sub-optimal vision (20/40) related to a poor tear film despite aggressive therapy, but the patient is less symptomatic?
Management of ocular surface disease is largely based on patient symptoms. However, the tear film plays an important role in maintaining ocular health. Increasing medical therapy for a symptomatic patient is easy. The challenge is getting a patient to accept additional therapeutic measures when he or she feels comfortable and sees well, despite having an ocular surface that is at risk.
So, when we ask why dry eye therapy fails, we must recognize that treatment goals or thresholds after a patient has become asymptomatic have not been clearly defined. Until such a time, success or failure in dry eye management is a relative concept.
Meibomian gland dysfunction is one etiology of dry eye. What do you do when your treatment options dont work? Photos courtesy: Barbara Caffery, O.D.
With this in mind, we will address five common reasons why management of ocular surface disease sometimes falls short of a doctors or patients expectations.
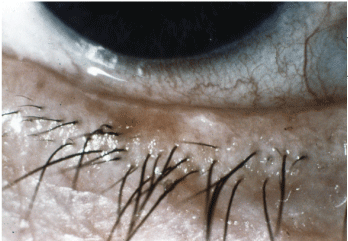
Delayed Diagnosis and Treatment
According to the Womens Health Study and Physicians Health Study, an estimated 3.2 million women and 1.05 million men past age 50 suffer from clinically significant dry eye.1 Other prevalence data, including the Beaver Dam Eye Study and Salisbury Eye Evaluation (SEE) Project suggest that these numbers may be much higher.2-5 When you consider that pharmacists across the country make more than 600,000 recommendations per month on artificial tear products alone, it becomes apparent that dry eye is a grossly under-diagnosed condition.6
Oliver Schein, M.D., and colleagues, determined that the average dry eye patient suffers with symptoms related to his or her disease for 6.5 years before seeking care from an eye-care practitioner.3 Furthermore, 76% of those patients surveyed reported a worsening of their symptoms during that time. Many individuals, who likely self-medicate with OTC products, are unaware that ocular surface disease is a progressive disease that, if left unchecked, can result in chronic ocular discomfort, loss of vision and a decreased quality of life. The longer the condition goes undiagnosed, the greater the challenge in managing it.
Incorrect Diagnosis or Treatment Plan
A 60-year-old female presents for a second opinion complaining of burning and scratchiness that has persisted for six months. During your initial conversation, you notice a rapid blink rate in the presence of normal interpalpebral fissures and complete lid closure. What stands out most is the red rash at the central axis of her brow and face.
When asked about recent treatments for her irritated eyes, she indicated that she called her eye doctors office four months ago. The employee who took the call recommended using Optive Lubricant Eye Drops (Allergan). The patient has used Optive q.i.d. since then, with little improvement in her symptoms. Assuming that this is all that her doctor would recommend had she made an appointment, she decided to come to you for a second opinion.
On slit-lamp examination, you notice a rather frothy tear film, as well as lid margin telangiectasia. Transillumination of the lower lids shows no evident meibomian gland dropout, so you decide to treat this meibomianitis (secondary to acne rosacea) systemically with low-dose doxycycline (LDD) 20mg b.i.d. You discuss the potential side effects of LDD, including gastrointestinal (GI) upset, and offer strategies to minimize these side effects. Also, you emphasize the need to avoid antacids or dairy products, as they can render the medication ineffective.
Your patient returns one month later and reports mild improvement in her symptoms. She said she complies with using the warm compresses and LDD, and examination shows some improvement in her lid injection. However, expression of her meibomian glands suggests they are still congested. You ask her if she has experienced any GI upset. She admitted, at first, that she did; but, now she finds relief by taking Tums (GlaxoSmithKline).
It is important to establish a dry eye protocol for you, your staff and your practice. In this example, an innocent recommendation offered over the phone by a staff member resulted in an established patient choosing a second opinion. The other doctor was unaware that the patient had even called his office.
Treatment plans are less likely to be incorrect after a thorough case history and dry eye work-up. Usually, one of a few valid treatment strategies is recommended based on the severity of symptoms, objective clinical findings, patient goals and likely adherence barriers.
There is no cookbook strategy that is the best for every patient; however, efforts set forth by the International Task Forces Delphi Panel, followed by the International Dry Eye Workshop, in developing a treatment algorithm, have been invaluable. But, I think each of the experts on these panels would remind us that aqueous deficient or inflammatory dry eye does not exist in a vacuum. Significant disease co-morbidity exists within our dry eye patient base.
Incorrect treatment plans occur because a formal assessment is simply not done. Usually, it is because dry eye complaints are often treated as an issue of lesser importanceone warranting an artificial tear sample from the cabinet with instructions to call if symptoms do not improve.
To determine the impact of dry eye disease on quality of life, a group of researchers asked patients: How many years would you give up from the end of your life to be symptom-free now?7 Patients responses were scaled from 0 to 1, with 0 being death and 1 being perfect health. Responses for moderate (0.78) to severe (0.72) dry eye were similar to historical reports for moderate to severe angina, with more severe cases of dry eye disease (i.e., requiring tarsorrhaphy) having a lower or worse utility scorecomparable to a disabling hip fracture.
Make sure your entire staff understands that dry eye is a separate disease entity that is multifactorial in nature and can have a profound impact on a patients quality of life. Any complaints that are characteristic of ocular surface disease must be formally assessed. Establishing protocols to address this need will undoubtedly make you more successful in its management.
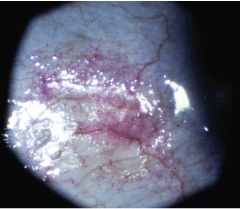
Rose bengal staining of the bulbar conjunctiva reveals the presence of dry eye syndrome in another patient.
Treatment Costs as a Barrier to Both Patient and Doctor
A 68-year-old black female is referred to you from the local glaucoma specialist for management of her dry eye complaints. The specialist reports that this patient is on Lumigan (bimatoprost, Allergan) q.h.s., Alphagan P (brimonidine tartrate, Allergan) b.i.d., and Refresh preservative-free tears (Allergan).
The glaucoma specialist also reports that the patient had a relatively successful trial of Restasis, but for some reason she no longer uses it. Upon further questioning, the patient indicates that she was enrolled in a patient assistance program, which enabled her to get the glaucoma and dry eye medications she needed. But, when she signed up for Medicare Part D, she automatically lost her eligibility for patient assistance. Furthermore, her Medicare Part D plan does not cover any of the medications she currently takes. She admitted that she is on a fixed income and that the cost of her glaucoma drops alone causes financial hardship. There was no way she could afford the Restasis.
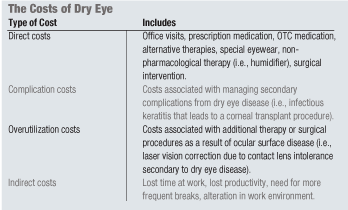
Direct medical costs associated with managing ocular surface disease (OSD) can be a burden, especially when a patient may already be overextended financially with other health issues. As this case shows, patients often are forced to prioritize their health problems and decide how to best use their limited prescription drug plans and financial resources.
Being sensitive to this burden, physicians may delay more costly, yet needed, treatments or pre-judge patients as to their ability to afford such treatments. As treating physicians, it is important to remember that it may ultimately cost the patient more in the long runvia complication costs, overutilization costs and other indirect costsby not aggressively treating their ocular surface disease at the earliest opportunity. (See The Costs of Dry Eye.)
For example, Warren Cross, M.D., discussed the positive impact Restasis had on 181 of his dry eye patients.8 The benefits realized included:
Fewer co-pays due to fewer office visits. Some patients were able to cut their visits down from monthly to bi-annually.
Less time off from work to make these follow-up visits.
Improved productivity at work, as symptoms had significantly improved.
Decreased need for punctal occlusion.
Significantly decreased usage of OTC artificial tears, with some discontinuation.
Take the time to inform your patients about the impact that dry eye disease may have in terms of indirect costs and quality of life. Emphasize that your goal is to keep their direct and indirect costs as low as possible. This way, patients will be more likely to adopt and stick with your treatment plan.
Non-Adherence or Compliance
Whether we are talking about contact lens wear or the management of glaucoma, we have all seen treatment plans poorly followed. With respect to dry eye disease, there is no question that overall adherence is better when patients are symptomatic, either physically or visually. The real challenge lies when their treatment regimen is working, and patients become asymptomatic.
L. Osterberg, M.D., and colleagues summarized the major predictors of poor medication adherence based on a review of multiple clinical studies.9 Several of these predictors apply to the management of ocular surface disease, including:
The treatment of a chronic disease.
The treatment of an asymptomatic disease (often intermittent with OSD).
Inadequate follow-up or discharge planning.
Medication side effects.
Patients lack of insight into the illness.
Poor provider-patient relationship and communication.
Missed appointments.
Complexity of treatment.
Cost of treatment.
Understandably, there are similar barriers specific to eye drop compliance. (See Forms of Drop NonCompliance.) In fact, one study identified more than 70 different compliance barriers in the management of glaucoma.10
We need to employ strategies that will help our patients overcome these barriers. The key is establishing a cooperative doctor-patient relationship in which an informed patient actively participates in his or her own care. In doing so, the patient is more likely to bring potential obstacles to carrying out your proposed treatment plan to your attention.
Failure to Establish Realistic Expectations
A 55-year-old female with chronic dry eye asks for an appointment, saying her dry eye problems are back. She has been doing wonderfully for the past 10 months since you added Restasis (cyclosporine, Allergan) to her preservative-free tear regimen more than a year ago.
She presents with an increase in corneal and conjunctival staining and an intermittent one-line drop in best-corrected visual acuity, and her score on her Ocular Surface Disease Index (OSDI) has gone from 22.7 to 62.5. She also reports an increase in thirst and water consumption.
You inquire about any recent changes in her medications, including OTC products. You also ask about changes in diet, including alcohol or caffeine consumption. Does she have any new pets, or is she using any new makeup that could be acting as an irritant?
After exhausting the list of environmental, general health and lifestyle risk factors, you decide to increase her level of treatment. You also consider ordering blood work to rule out autoimmune disease as an underlying cause for her decline.
As she is leaving, she mentions that her husband recently installed an old-fashioned wood-burning stove to reduce their heating costs. She asks whether that might have anything to do with her worsening condition.
Despite therapeutic advances, ocular surface disease remains a recalcitrant condition. It is important to educate your patients from the beginning that there are extrinsic and intrinsic variables that, at any time, can lead to a worsening of symptoms. These include:
Environmental conditions, such as windy or arid climates, airborne pollutants or smoke.
Systemic conditions, which require medications that may have a drying effect, including hypotensives, anti-depressants, and allergy medications.
Concomitant ocular disease, such as glaucoma.
Even in the absence of these risk factors, managing inflammatory dry eye can take time before results are seen, and symptoms may still wax and wane, even in the most controlled environments. Failure to bring this to your patients attention early on may cause them to discontinue treatment.
Realistic expectations about the variant nature of OSD are not only important for the patient, but also for the treating physician. It is natural to get discouraged when, despite your best efforts, patients are either slow to respond, or they backslide in their progress. It is equally exhilarating, however, when your Sjgrens syndrome patient tells you she can leave the house on a windy day without fear of the pain that she used to experience.
Management of ocular surface disease can be a roller coaster ride for you and your patients. But, if you educate your staff on the importance of formally addressing a patients symptoms and follow up with some simple protocols for your practice, you will improve your patients overall quality of life, and you will establish a very loyal following.
Dr. Mangan, a graduate of the Illinois College of Optometry, is chair of the refractive surgery and clinical research committees for the Eye Center Group, a multi-specialty comanagement center in central
1. Schaumburg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003 Aug;136(2):318-26.
2. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000 Sep;118(9): 1264-8.
3. Schein OD, Hochberg MC, Muoz B, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med 1999 Jun 28;159(12):1359-63.
4. Schein OD, Muoz B, Tielsch JM, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol 1997 Dec;124(6): 723-8.
5. Muoz B,
6. 2008 Survey of Pharmacists Recommendations for OTC Products. Supplement to Pharmacy Times, June 2008.
7. Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology 2003 Jul;110(7):1412-9.
8. Cross WD, Lay LF Jr, Walt JG, Kozma CM. Clinical and economic implications of topical cyclosporin A for the treatment of dry eye. Manag Care Interface 2002 Sep;15(9):44-9.
9. Osterberg L, Blaschke T. Adherence to medication.
10. Tsai JC,
11. Lee MD, Fechtner FR, Fiscella RG, et al. Emerging perspectives on glaucoma: highlights of a roundtable discussion. Am J Ophthalmol 2000 Oct;130(4 Suppl):S1-11.

