The foundation of optometry is evaluating a patient’s visual status by asking, “Which is better, 1 or 2?” The goal is usually to achieve 20/20, or close, as their best-corrected visual acuity (BCVA). When a patient has reduced vision, it is our mission to determine the cause. Sometimes we discover significant pathology even when patients are correctable to 20/20. It is not unusual for these patients to have symptoms of visual distortion, glare, halos, fluctuating vision, ocular discomfort and headaches.
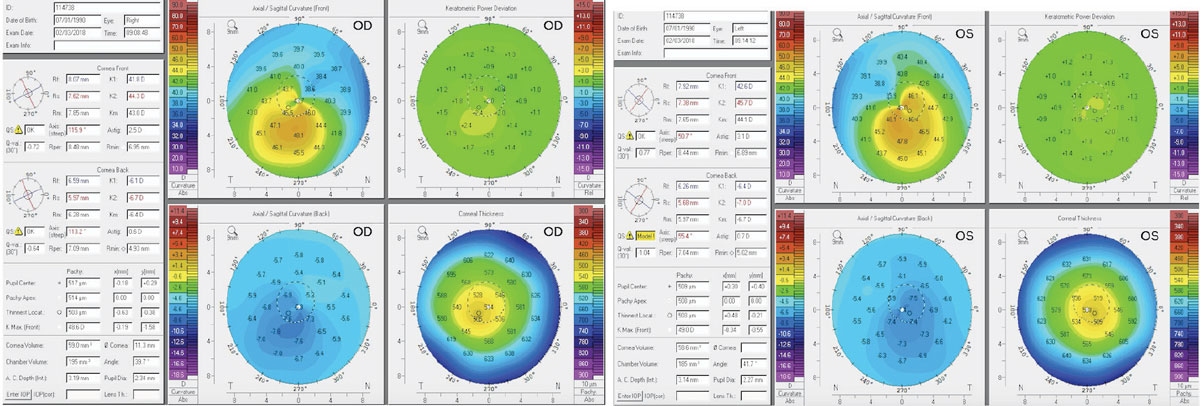 |
Figs. 1 and 2. Above, these corneal topography scans show a patient with subclinical keratoconus. The patient here was refractable to 20/20 but complained of excessive glare and halos. Note the inferior steepening and the thin central corneal thickness. Click image to enlarge. |
Here, we review conditions where a patient may be correctable to 20/20, but could be ultimately diagnosed with ocular pathology. If these conditions are not detected, there could be significant, long-term visual or systemic consequences.
Visual acuity measured with a simple pinhole occluder will help determine if a refractive change is likely to improve vision. We will discuss supportive ancillary testing such as corneal topography, color vision testing and optical coherence tomography (OCT), as well as indications of when to use technology to diagnose ocular pathology when the primary visual symptom or findings is not decreased BCVA.
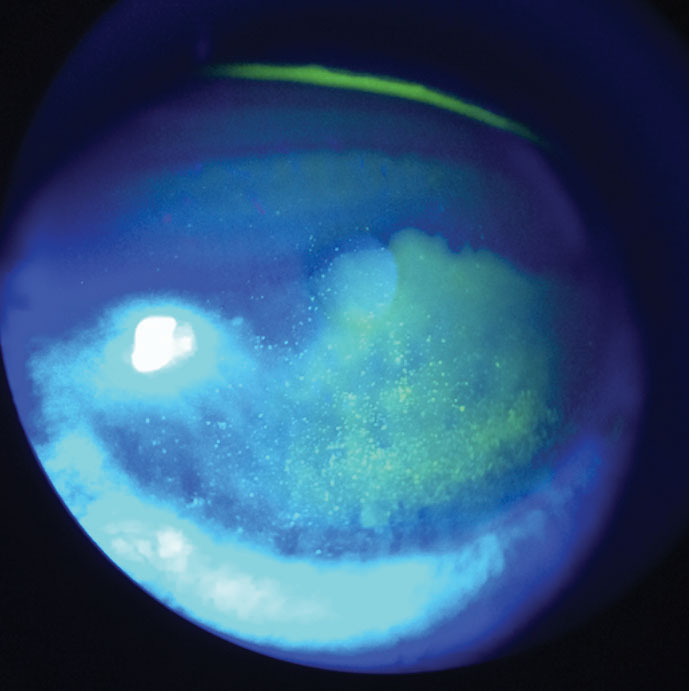 |
| Fig. 3. At left, this patient demonstrates diffuse corneal staining with sodium fluorescein due to toxicity from multipurpose contact lens solution. Click image to enlarge. |
Cornea
Keratoconus is a progressive corneal ectasia characterized by a weakening and thinning cornea.1 While the vast majority of keratoconus presents bilaterally, doctors find a high prevalence of asymmetry between the eyes. Research from 2010 defines forme fruste keratoconus as the contralateral eye in unilateral keratoconus without any ectatic changes.2
In mild cases, such as subclinical keratoconus or forme fruste keratoconus, no obvious biomicroscopy signs—such as apical scarring, Vogt’s striae, Fleisher’s ring or Munson’s sign—may present (Figures 1 and 2). These patients may be refractable to 20/20, but still report symptoms of debilitating glare, ghost image, pseudodiplopia and a history of active eye rubbing. For these patients, central keratometry readings measured by standard autorefractors are often misleading.
To help ensure early and accurate detection, corneal topography is critical. Specifically, a corneal thickness map and the difference in keratometry readings between the thinnest and thickest areas may be required. Patients with forme fruste keratoconus will have a lower corneal thickness and a higher keratometric differences compared with normal eyes.3
Glare testing and contrast sensitivity can be important diagnostic testing for early forme fruste patients. A 2016 study found that contrast sensitivity was more affected and is a more sensitive indicator of visual function in keratoconus compared with BCVA.4 Fortunately, form fruste keratoconus tends to progress slowly, if at all. But the chronic symptoms may still affect a patient’s quality of life. Therefore, proper diagnosis and management can have a significant effect on a patient’s functional vision.
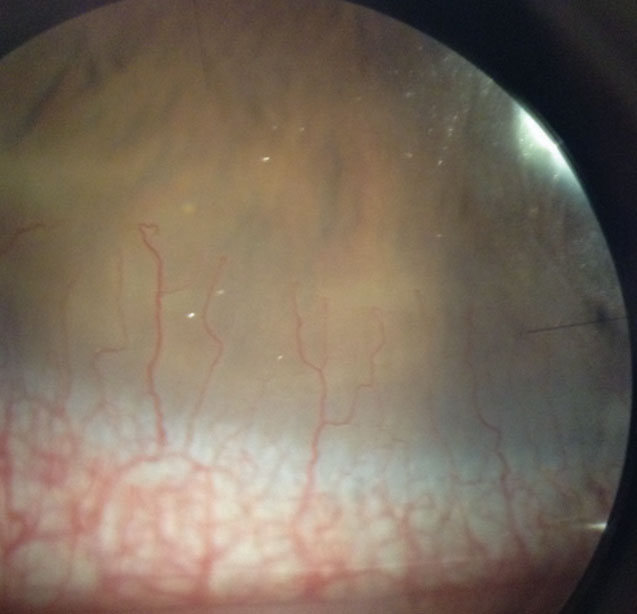 |
| Fig. 4. This asymptomatic patient’s corneal neovascularization is due to contact lens overwear. Click image to enlarge. |
Contact Lenses
Optometrists are well aware that bacterial corneal ulcers from contact lens overwear can be devastating to a patient’s vision. In cases of acute bacterial keratitis, we immediately advise a patient to discontinue contact lens wear. These conversations must be initiated when patients have signs of contact lens overwear that have not yet affected vision, even if they are asymptomatic.
A variety of ocular findings are associated with contact lens overwear that may present prior to decreased visual acuity but may affect the quality of a patient’s vision, as well as increase that patient’s risk for more serious complications. For example, even when a patient is correctable to 20/20 and asymptomatic, we should evert the upper eyelid to evaluate the superior palpebral conjunctiva and use sodium fluorescein to check for corneal integrity due to associated risks of new ocular symptoms and complications (Table 1).
Often, patients will continue to experience 20/20 visual acuity but have visual complaints regarding the quality of their vision when they undergo punctate epithelial keratopathy (PEK) related to contact lens wear or dry eye. Any contact lens wear can potentially affect the integrity of the corneal surface and cause damage to corneal epithelial cells.
The most common and convenient clinical method for visualizing and diagnosing corneal damage is with the use of sodium fluorescein.5 The extent of corneal staining from contact lens wear can vary depending on the degree of insult. Diffuse superficial staining covering the entire cornea can result from toxicity to preservatives in multipurpose solutions (Figure 3). Patients with this low-grade staining may not complain of any blurred vision, ocular discomfort or dryness.5
Contact lens wear can also compromise optical clarity by causing corneal edema, which is a hypoxia-related corneal complication. Patients with microcystic edema from contact lens wear often only complain of halos and glare around lights (Sattler’s veil). At times, subtle edema may not be easily visualized on slit lamp examination.
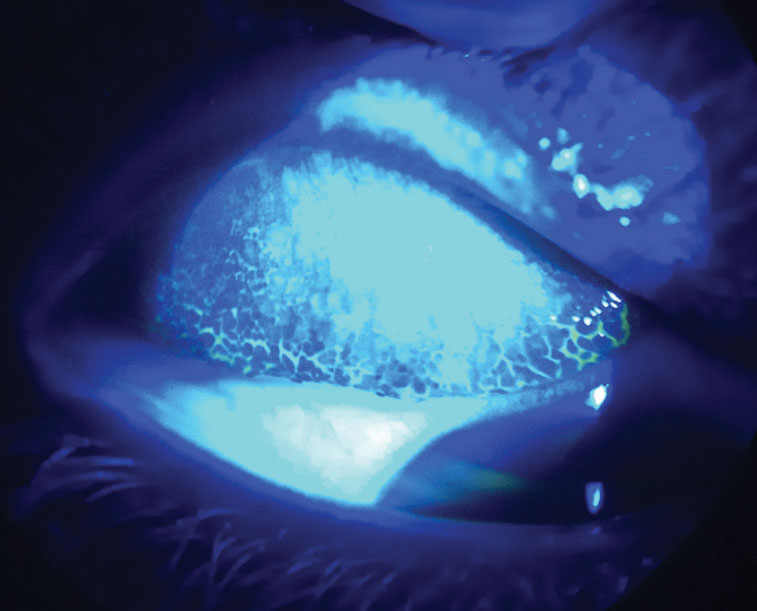 |
Fig. 5. By everting this patient’s upper lid and using sodium fluorescein dye with a cobalt blue light, you can see their giant papillary conjunctivitis from contact lens overwear. Click image to enlarge. |
Measuring central corneal thickness with pachymetry or measuring corneal density with instruments such as Scheimpflug imaging via Pentacam (Oculus) and anterior segment OCT (AS-OCT) may also be useful to assess the full impact of contact lens wear on the cornea.6
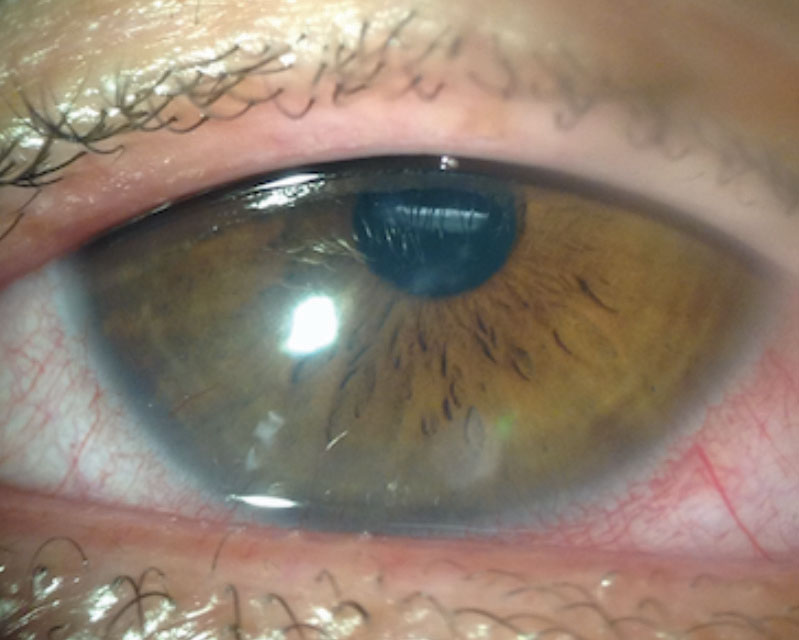
Corneal neovascularization (CNV) can result from contact lens wear, corneal infections or corneal inflammatory conditions (Figure 4). When hypoxia stimulates vessel growth from the pericorneal vascular plexus into the cornea, the result is new vascularization in an otherwise clear structure.7
A Japanese research team classified CNV based on grades of severity. Grade 1, the least severe, involves only the peripheral cornea. Grade 2 indicates peripheral and mid-peripheral involvement. Grade 3 signifies modest vascularization involving the entire cornea. Grade 4 is the most severe and indicates massive vascularization covering the entire cornea.8
Many patients with mild CNV are asymptomatic with 20/20 visual acuity. More severe, visually significant CNV associated with contact lens wear is less common with high oxygen permeable silicone hydrogel materials, especially daily disposable modalities. However, it can still occur and progress, especially if the contact lenses are abused and overworn.
Giant papillary conjunctivitis (GPC)—or contact lens–induced papillary conjunctivitis (CLPC)—is a potential complication of contact lens wear. Patients may present with 20/20 vision but often complain of redness, itching, contact lens intolerance, excessive lens movement and mucous discharge.9 GPC prevalence was significantly higher with conventional hydrogel contact lenses.9
 |
| Fig. 6. This patient’s anterior segment evaluation shows a case of CLARE. Click image to enlarge. |
We see less severe GPC with frequent replacement and daily disposable lenses, especially silicone hydrogel materials, which attract more lipid than protein deposits. Therefore, many practitioners may not look for GPC when a patient is 20/20. The definitive way to diagnose GPC is by everting the eyelids to visualize hyperemia and a papillary reaction in the palpebral conjunctiva. Instilling sodium fluorescein and viewing the upper tarsal conjunctiva with cobalt blue light can enhance the view of the papillary reaction (Figure 6). Other concurrent allergic and atopic conditions can increase the risk of developing GPC.
Contact lens–induced acute red eye (CLARE) is an inflammatory response to contact lens wear more often seen in patients who overwear their lenses. Patients may complain of end-of-day redness, discomfort or contact lens intolerance, glare and photophobia without any blurred vision. Biomicroscopy generally reveals circumlimbal or diffuse conjunctival injection.10 CLARE often resolves without topical medications by discontinuing contact lens wear temporarily, or decreasing the hours of contact lens wear. Additionally, switching to higher oxygen permeable silicone hydrogel materials can also reduce symptoms of CLARE. However, if patients continue to overwear their lenses, mild CLARE may progress to more sight-threatening conditions such as infectious keratitis.
Contact lens–associated corneal infiltrative events (CIEs) are self-limiting inflammatory responses that indicate a host’s immune response to infection or an immune response to antigens (Figure 7).11 CIEs are often used to describe a sterile keratitis. However, investigators found that when cultured, sterile infiltrates are often positive for bacteria.12
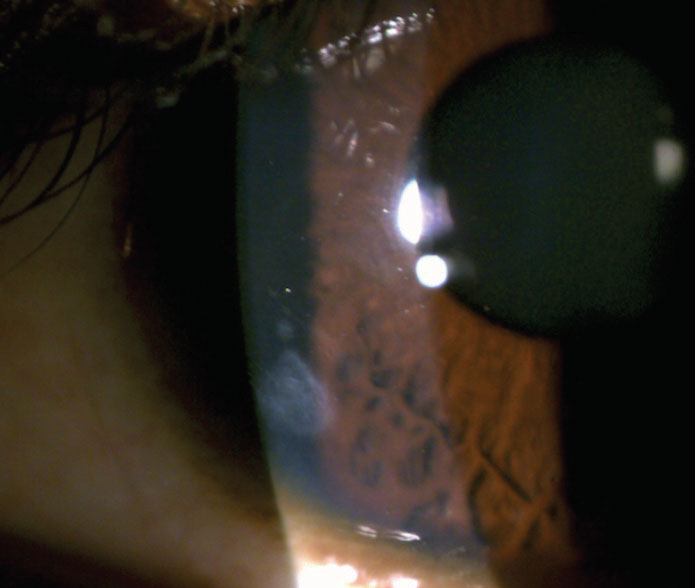 |
Fig. 7. You can see this patient’s residual peripheral corneal scar from a resolved contact lens–associated corneal infiltrative event. Click image to enlarge. |
Exotoxins released by gram-positive bacteria such as Staphylococcus aureus can cause migration of inflammatory cells, including polymorphonuclear leukocytes, which leads to infiltrate formation.12 Young male patients may be more likely to develop contact-lens–related complications due to poor compliance and greater risk-taking behaviors, according to some research.13 The incidence of CIEs in daily soft contact lens wearers is approximately 3%.11 When reviewing extended wear patients, the incidence of symptomatic CIEs ranges between 2.5% to 6% and, when including asymptomatic CIEs for extended wear, the incidence increases dramatically to 20% to 25%.11
Unlike CIEs, contact lens peripheral ulcers (CLPU) are focal excavations of the epithelium, which can cause infiltration and necrosis of the anterior stroma near the corneal margins. Patients with CLPU may be asymptomatic with 20/20 visual acuity or may complain of redness, pain or foreign body sensation. Microbial keratitis (MK) can be visually significant and involves actively proliferating bacteria, which requires treatment.11
 |
| Click image to enlarge. |
Dry Eye Disease
Perhaps one of the more common types of patients with 20/20 vision and concurrent complaints are those with dry eye disease (DED). In the Tear Film and Ocular Surface Society’s Dry Eye Workshop report from 2017, dry eye is defined as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiologic roles.”14
This thorough and complex definition reflects the variety of challenges we may have managing these patients. Individuals suffering from DED may vary from only having mild symptoms and signs to severe ocular surface complications.
To identify patients at risk for DED and associated ocular symptoms such as fluctuating vision, light sensitivity and unstable refractions, start by asking triaging questions and identifying risk factors such as smoking, contact lens wear or at-risk medications. A dry eye survey such as the Ocular Surface Disease Index (OSDI) or the Dry Eye Questionnaire-5 (DEQ-5), due to its short length and discriminative ability, can be given prior to the examination.14,16 Additionally, for soft contact lens (SCL) patients, a preferred survey would be the eight-item Contact Lens Dry Eye questionnaire (CLDEQ-8), which has repeatable results and comes closest to identifying SCL wearers who would likely benefit from clinical management of dry eye.17,18
When patients are identified early with DED, additional attention may be warranted during refraction to assess for consistency of responses, as the tear film is the first refractive component of the eye. When asked to blink during refraction and visualize the changes in visual quality, patients often recognize the contribution of DED to their symptoms and may be more apt to follow through on therapy.
 |
| Click image to enlarge. |
Systemic Medications
Many systemic medications can impact vision—some of which may not directly affect acuity but can cause changes in color vision, quality, transient visual obscurations or other atypical symptoms (Table 2). When patients have complaints that are new or different—even if they have 20/20 BCVA—consider whether any of the patient’s medications is likely to play a role.
One ocular condition associated with many medications is idiopathic intracranial hypertension (IIH), historically referred to as pseudotumor cerebri. It may be associated with tetracycline, doxycycline, minocycline, corticosteroids, oral contraceptives, amiodarone, tamoxifen, levothyroxine and high-dose vitamin A therapy. These patients often present early in the condition with good acuity but with headaches, possible double vision and pulsatile tinnitus.
Due to the finding of bilateral optic nerve head elevation customarily observed with IIH and the high—risk differential of true papilledema, patients with this finding warrant immediate referral for brain and orbit imaging followed by a lumbar puncture to rule out increased intracranial pressure.
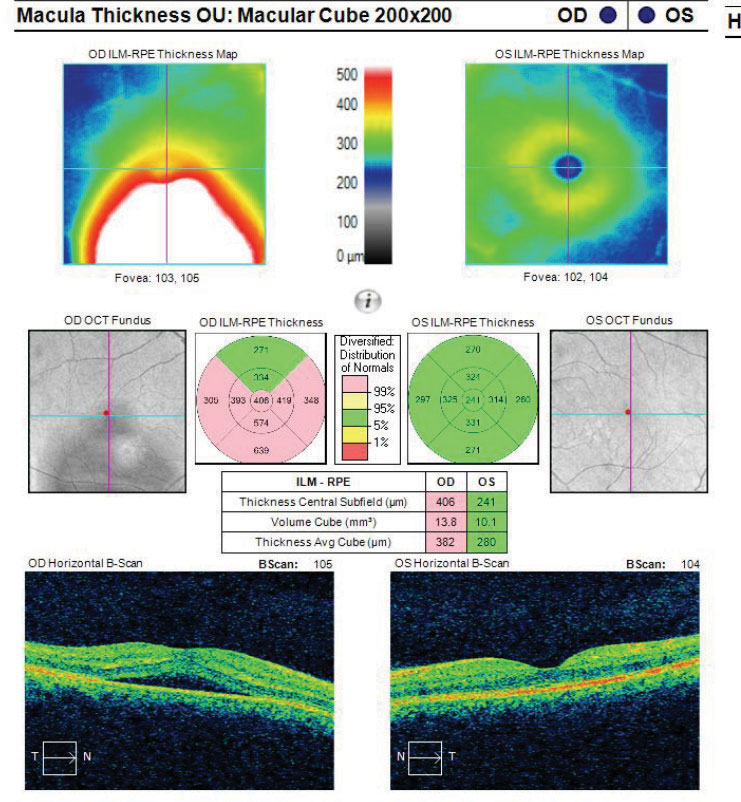 |
Fig. 8. This macular cube image demonstrates a patient with RPE disruption with CSCR. Click image to enlarge. |
Patients may complain of a blurred or distorted area in their vision and still have 20/20 BCVA and have a concurrent scotoma that affects the paracentral field. In this case, visual field testing may be especially helpful.
Visual field assessments ranging from threshold automated visual fields to Amsler grids may be beneficial in identifying and targeting defects. Additionally, in cases where blur or distortion is reported, it is important for the doctor to personally assess the individual’s demographic status, review systemic medications, personality type and general observations. For example, an elderly patient with a history of smoking and a complaint of distortion is more likely to have complications associated with age-related macular degeneration to explain the scotoma whereas a younger, highly stressed individual may be more likely to have a diagnosis of central serous chorioretinopathy (CSCR).
Central Serous Chorioretinopathy
These patients nearly always report a spot in their vision but, depending on the location of the scotoma, may have decreased BCVA or may retain 20/20 visual acuity.
Though the exact etiology for CSCR is unknown, suspected associations include type-A personalities, corticosteroid use, pregnancy and a variation associated with optic nerve pits.19-23 For patients using corticosteroids and developing CSCR, it appears that both the choroid and retinal pigment epithelium (RPE) are involved (Figures 8 and 9). One proposed hypothesis is that some patients, due to idiosyncratic susceptibility of their individual mineralocorticoid pathway, may be more susceptible to CSCR while using steroids.
A detailed review of medications and history by the eye care practitioner is important for these patients as the types of steroids associated may vary widely—anywhere from oral steroids used to treat facial palsy, systemic lupus erythematosus or ulcerative colitis to steroid inhalers, epidural steroid injections and even dermatological/topical steroid use.19-22
When CSCR is suspected, an OCT of the area of elevation and associated tissues may be beneficial for a variety of reasons. Not only can an OCT help confirm the diagnosis of CSCR but it may also rule out other high-risk differentials and comorbidities (e.g., choroidal neovascular membrane).
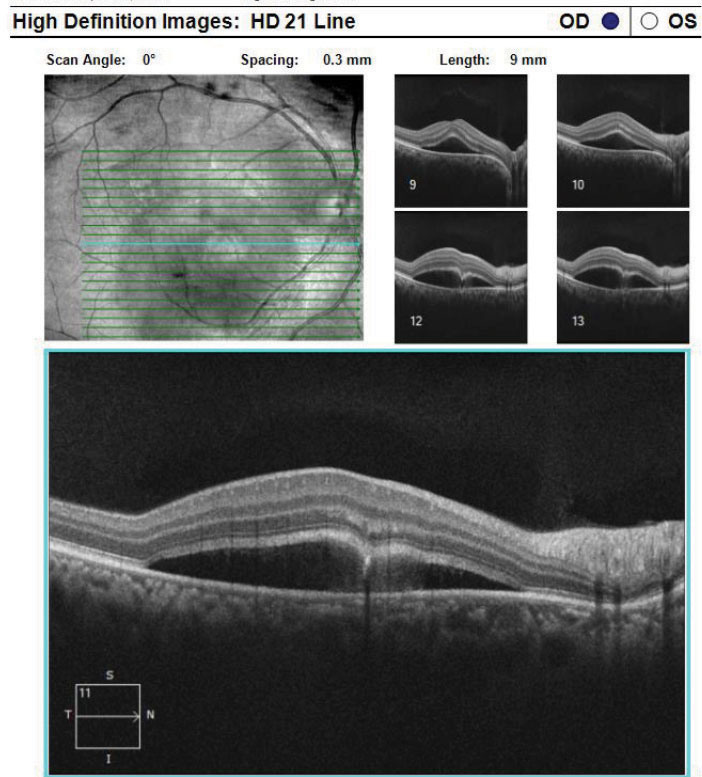 |
| Fig. 9. This is a high-definition 21-line raster of the RPE disruption with CSCR from the same patient above. Click image to enlarge. |
Additional assistance from OCT measurements can help in understanding possible underlying associations with a diagnosis of CSCR.23 In 2017, investigators described differences in OCT measurements of the choroid between eyes with CSCR associated with steroids vs. acute idiopathic CSCR during the acute phase.23 The researchers measuremed between the outer border of the RPE and the chorioscleral border using SD-OCT and found that patients suffering with CSCR associated with steroids have significantly thinner OCT choroidal measurements than those with acute idiopathic CSCR.23 In those with a steroid association, communication with both the patient and any managing physicians is key in reducing the patient’s systemic “steroid” load and allowing the CSCR to resolve.
Initial management of patients with CSCR may include reassurance and observation or stress relief management, modifications of medications (steroids) for patients with associations and regular dilated examinations (three to six weeks) until resolution. However, for patients with chronic (persistent subretinal fluid for three to six months) or recurrent CSCR, various therapeutic options exist—from short-duration, multi-session subthreshold micropulse yellow laser to photodynamic therapy with vertoporfin to oral aldosterone antagonists.24-26
Even patients with 20/20 BCVA may have visual symptoms such as glare, halos, ocular pain, distorted vision, headaches and may also show signs of pathology while being asymptomatic—all concerns that warrant additional care. Patients with good vision may ultimately have other conditions such as keratopathies, maculopathies and optic neuropathies which require management. As eye care specialists, we can use technology to assist our patient’s well-being. For example, corneal topography and OCT are now common equipment in many practices. Clearly, the integration of technology can change the way we diagnose and manage our patients. Assessing visual acuity is only a small part of what we are capable of. These examples show that refracting a patient to 20/20 is not always enough.
Dr. Tyler is an associate professor and module chief of primary care for The Eye Care Institute at Nova Southeastern University in Davie, FL.
Dr. Nguyen is an assistant professor at Nova Southeastern University in Davie, FL.
| 1. Zhao F, Du F, Zhang J, et al. Trends in research related to keratoconus from 2009 to 2018: A bibliometric and knowledge mapping analysis. Cornea. 2019;38:847-54. 2. Saad A, Gatinel D. Topographic and tomographic properties of form fruste keratoconus corneals. Invest Opthalmol Vis Sci. 2010;51:5546-55. 3. Awad E, Abou Samra W, Torky M, El-Kannishy A. Objective and subjective diagnostic parameters in the fellow eye of unilateral keratoconus. BMC Ophthalmology. 2017;17(1):186. 4. Bilen N, Hepsen I, Arce C. Correlation between visual function and refractive topographic, pachymetric and aberometric data in eyes with keratoconus. Int J. Ophthalmol. 2016;9(8):1127-33. 5. Fonn D, Peterson R, Wood C, et al. Corneal staining as a response to contact lens wear. Eye and Contact Lens. 2010;5:318-21. 6. Doughty M, Jonuscheit S. Corneal structure, transparency, thickness and optical density (densitometry), especially as relevant to contact lens wear – a review. Contact Lens and Anterior Eye. 2019;42(3):238-45. 7. Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and emerging therapies for corneal neovascularization. The Ocular Surface. 2018;16:398-414. 8. Inatomi T, Nakamura T, Koizumi N, et al. Midterm results on ocular surface re- construction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141(2):267–75. 9. Alipour F, Khaheshi S, Soleimanzadeh M, et al. Contact lens-related complications: A Review. J Ophthalmic Vis Res. 2017;12(2):193-204. 10. Sankaridurg P, Vuppala N, Sreedharan A, et al. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol. 1996;44(1):29-32. 11. Steele K, Szczotka-Flynn L. Epidemiology of contact lens induced infiltrates: an updated review. Clin Exp Optom. 2017;100:473-81. 12. Donshik P, Suchecki J, Ehlers W. Peripheral corneal infiltrates associated with contact lens wear. Trans Am Ophthalmol Soc. 1995;93:49–60. 13. Stapleton F, Naduvilath T, Keay L, et al. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS ONE. 12(8):e018343; 2017 14. Nelson J, Craig J, Akpek E, et al. TFOS DEWS II Introduction. Ocul Surf. 2017;15(3):269-75. 15. Wolffsohn J, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodoloy report. The Ocular Surface. 2017;15(3):544-79. 16. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55–60. 17. Chalmers R, Keay L, Hickson-Curran S, et al. Cutoff score and responsiveness of the 8-item Contact Lens Dry Eye Questionnaire (CLDEQ-8) in a large daily disposable contact lens registry. Cont Lens Anterior Eye. 2016;39(5):342-52. 18. Chalmers RL, Begley CG, Moody K, et al. Contact Lens Dry Eye Questionnaire-8 (CLDEQ-8) and opinion of contact lens performance. Optom Vis Sci. 2012;89(10):1435-42. 19. Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1984;68(5):329-31. 20. Fardin B, Weissgold DJ. Central serous chorioretinopathy after inhaled steroid use for post-mycoplasmal bronchospasm. Br J Ophthalmol. 2002;86(9):1065-6. 21. Lee SB, Kim JY, Kim WJ, et al. Bilateral central serous chorioretinopathy with retinal pigment epithelium tears following epidural steroid injection. Indian J Ophthalmol. 2013;61(9):514-5. 22. Chan LY, Adam RS, Adam DN. Localized topical steroid use and central serous retinopathy. J Dermatolog Treat. 2016;27(5):425-6. 23. Honda S, Miki A, Kusuhara S, et. al. Choroidal thickness of central serous chorioretinopathy secondary to corticosteroid use. Retina. 2017;37:1562–7. 24. Scarinci F, Ghiciuc C, Patacchioli F, et al. Investigating the hypothesis of stress system dysregulation as a risk factor for central serous chorioretinopathy: A literature mini-review. Curr Eye Res. 2019;44(6):583-9. 25. Kim YJ, Kim SY, Ha S et al. Short-duration multi-session subthreshold micropulse yellow laser (577nm) for chronic central serous chorioretinopathy: results at 3 years. Eye. (Lond). 2019;33(5):819-25. 26. Hanumunthadu D, Tan A, Singh S, et al. Management of chronic central serous chorioretinopathy. Indian J Ophthalmol. 2018;66(12):1704-14. |

