Accurate evaluation of the optic disc is a critical part of optometric practice. When a disc is not “perfused, healthy, distinct and flat,” it can be difficult to differentiate between anatomic variations and pathology. Clinicians must take a systematic approach to optic disc evaluation, carefully assessing the margins, color of the neuroretinal rim, cup-to-disc ratio and overall size of the nerve. This case-based review provides photos and clinical pearls to help you enhance your assessment of optic disc abnormalities.
 |
| Fig. 1. Despite unremarkable entrance testing with no visual complaints, the patient’s optic disc evaluation shows mild elevation with sectoral blurred margins nasally and superiorly OU. Click image to enlarge. |
Case 1: Surprise Elevation
A 43-year-old African American female presented for her annual exam without any visual complaints. Her health history was remarkable for hypothyroidism, asthma and iron-deficiency anemia. Entrance testing was unremarkable with 20/20 vision uncorrected OD and OS, full confrontation visual fields bilaterally and no relative afferent pupillary defect.
She exhibited 90% of normal abduction bilaterally with no diplopia and a 2 to 4 prism diopter comitant esophoria in all gazes. Her anterior segment exam was remarkable for palpebral conjunctival pallor, consistent with her history of anemia. Intraocular pressures (IOPs) measured 16mm Hg OD and 15mm Hg OS. Her blood pressure was elevated at 142/86mm Hg RAS. She was above her ideal body weight at 240lbs. Her dilated fundus exam revealed subtle elevation more so in the left eye than the right (Figure 1).
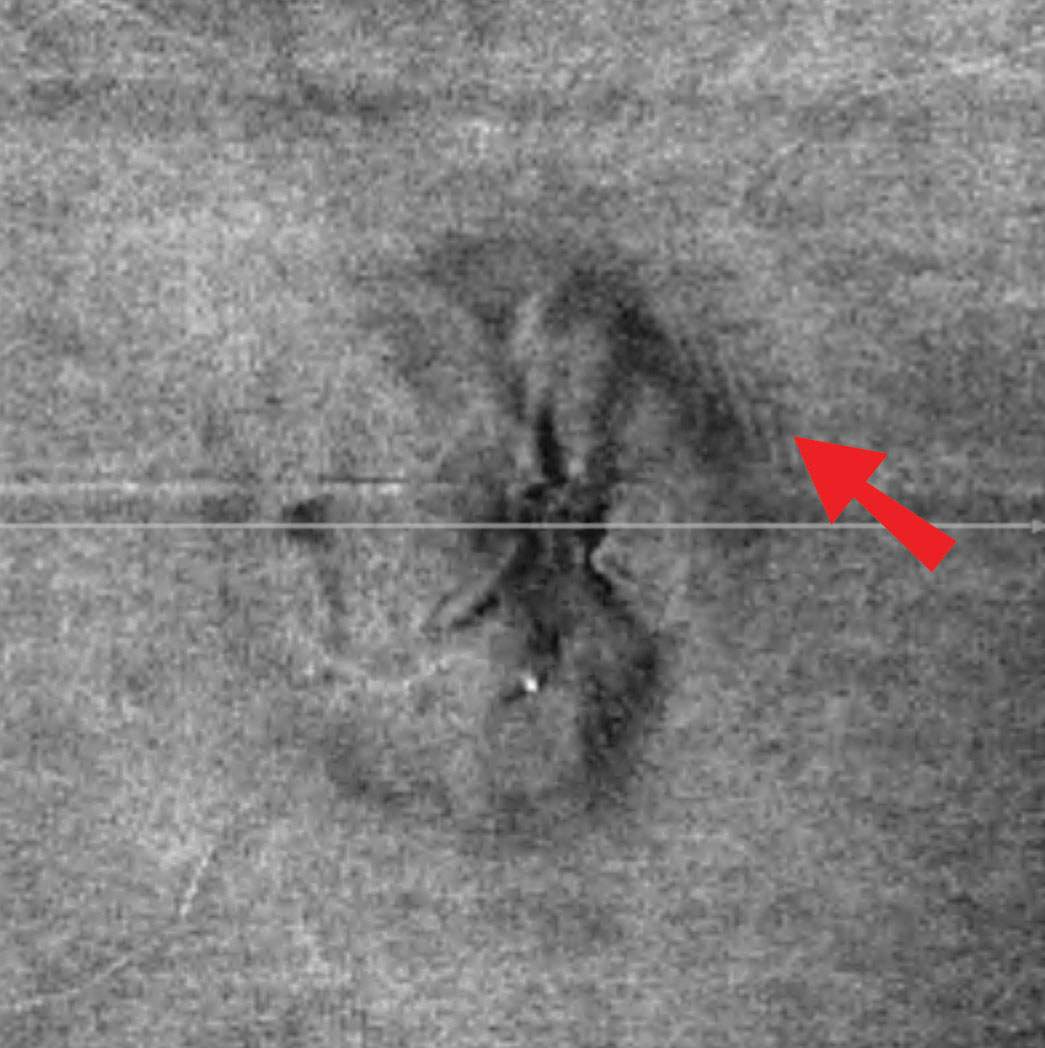 |
| Fig. 2. In this patient’s en face OCT vitreoretinal interface image of the left eye, note the wrinkles superior temporal, consistent with Paton’s lines or peripapillary wrinkles that are not readily visible funduscopically. Click image to enlarge. |
Discussion. The optic disc photos demonstrate elevation OS>OD, raising the question of papilledema vs. pseudopapilledema (Table 1). When asked about symptoms of increased intracranial pressure—such as headaches, pulsatile tinnitus, nausea, vomiting, diplopia and transient visual obscurations—the patient reported occasional headaches and “seeing stars” when she bent down. She denied any other symptoms or use of tetracyclines, vitamin A derivatives or oral contraceptives. She exhibited no spontaneous venous pulse (SVP). In addition, upon analysis of the vitreoretinal interface on optical coherence tomography (OCT), she demonstrated subtle peripapillary wrinkles in the left eye, suggestive of mild papilledema (Figure 2).
Due to her ocular findings, she was sent for urgent neuroimaging that included magnetic resonance imaging (MRI) of the brain and magnetic resonance venography of the head to rule out a mass and venous sinus thrombosis. MRI is the preferred neuroimaging modality due to superior soft tissue resolution and better visualization of particular findings consistent with intracranial hypertension such as optic nerve sheath distension, empty sella and posterior globe flattening.1 Imaging showed no evidence of intracranial mass or venous sinus thrombosis; however, she did exhibit low-lying cerebellar tonsils concerning for Chiari I malformation.
Table 1. Papilledema vs. PseudopapilledemaDifferentiating these clinical entities can be quite challenging. Causes of pseudopapilledema are relatively benign and include buried optic disc drusen and small, anomalous and/or hypoplastic discs. Papilledema is, by definition, optic disc edema in the setting of increased intracranial pressure and is a medical emergency. Several tests can aid in the evaluation:
|
Chiari 1 malformation is eight times more common in patients with pseudotumor cerebri than the general population, suggesting a relationship.2,3 The coexistence of Chiari 1 malformation and pseudotumor cerebri in patients with papilledema can create diagnostic and treatment dilemmas. In patients with papilledema, Chiari I malformation may be considered causative if there is obstruction of cerebrospinal fluid (CSF) flow.2,3
In this patient, lumbar puncture was deferred due to cerebellar ectopia; however, CSF flow was considered patent. Therefore, she was scheduled for follow-up with neurology on an outpatient basis for presumed idiopathic intracranial hypertension. Treatment was initiated with acetazolamide and weight loss was recommended.
The presence of peripapillary wrinkles on the vitreoretinal interface was important in raising the suspicion of papilledema on initial exam. Subsequent close optometric monitoring with assessment of the afferent system, dilated fundus exam and serial OCT scans is now indicated to assess the effectiveness of treatment and thus verity the working diagnosis.
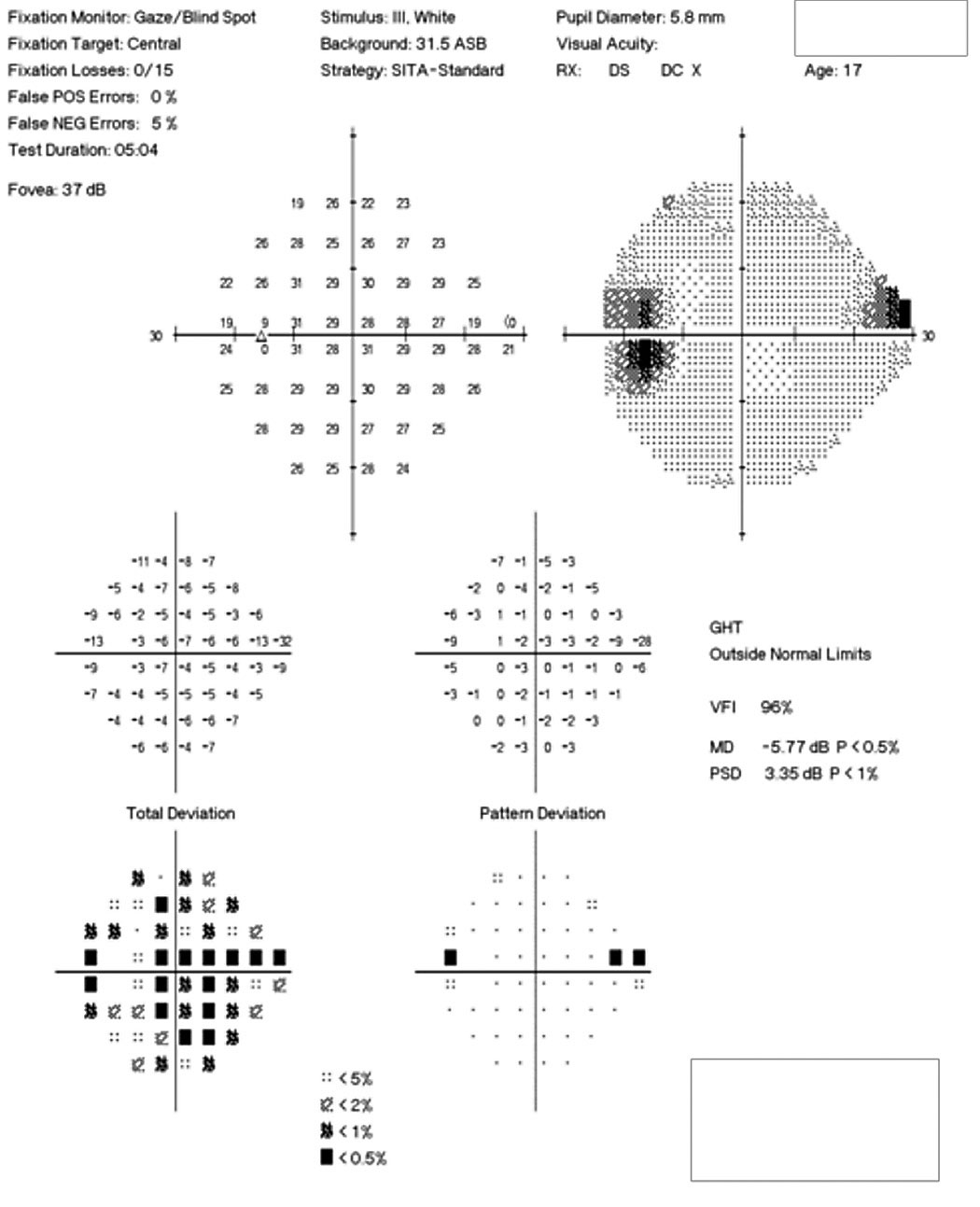 |
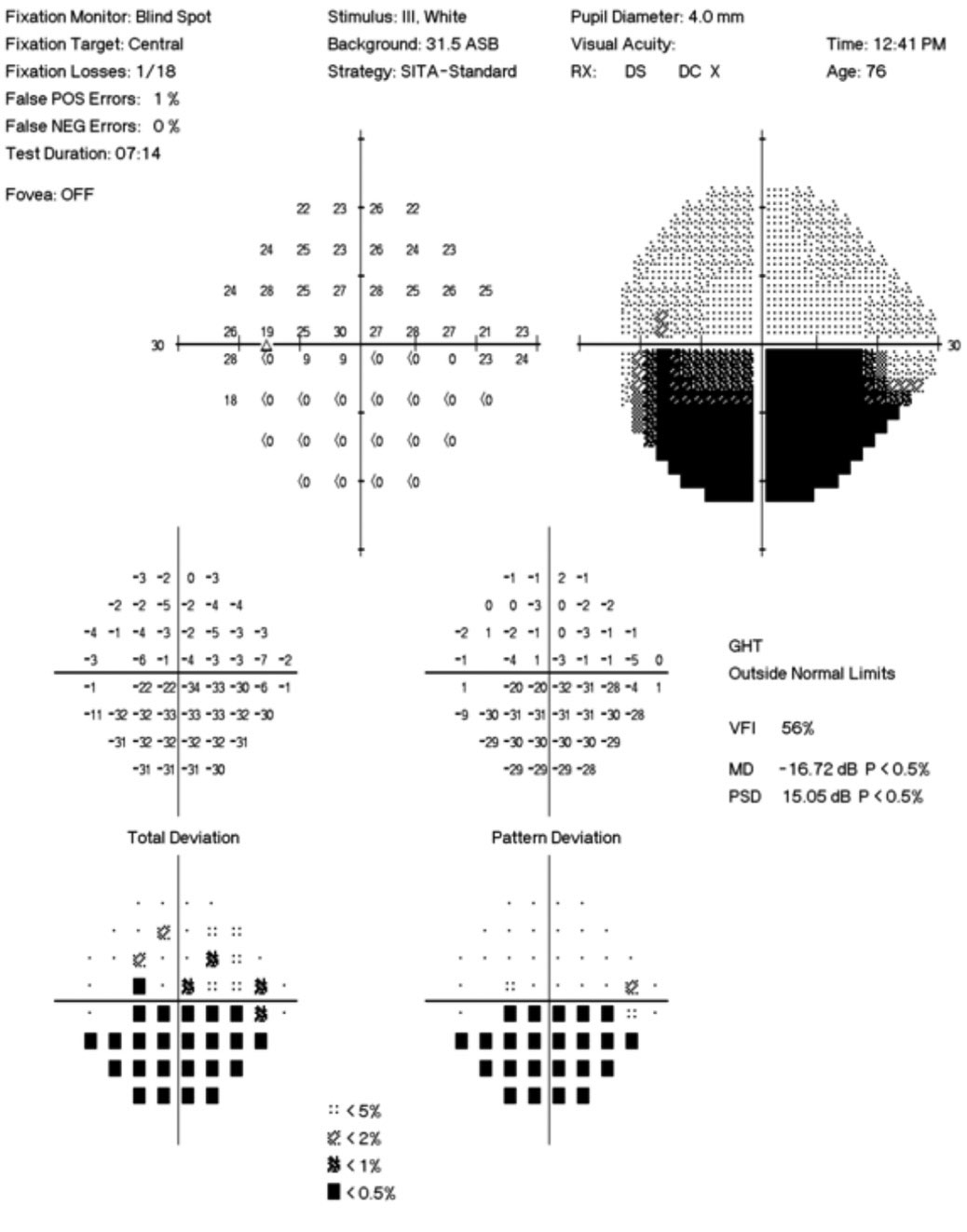
| Fig. 3. The patient’s 24-2 visual field OS demonstrates a small superior nasal step and enlarged blind spot. Click image to enlarge. |
Case 2: Problem Rising to the Surface
A 17-year-old Caucasian female presented for a routine examination. Her health history was unremarkable, and her only medication was oral contraceptives. She denied symptoms of increased intracranial pressure.
Her best-corrected visual acuity was 20/15 OD and OS. She exhibited a subtle, 0.3 log unit relative afferent pupillary defect in the left eye. Humphrey automated visual fields revealed a normal field in the right eye and a superior nasal defect with an enlarged blind spot in the left (Figure 3). Efferent testing was unremarkable with no abduction deficit. She exhibited no proptosis or ptosis. Pressures were 14mm Hg bilaterally.
Her dilated fundus exam revealed significant findings (Figures 4 and 5).
 |
| Click table to enlarge. |
Discussion. While also exhibiting indistinct margins of the optic disc as in case 1, this patient’s presentation was attributed to optic disc drusen. Fundus autofluorescence (FAF) was an important examination element in this case, as it helped to confirm the presence of superficial drusen in the left eye.
In the absence of drusen, the optic disc appears dark on fundus autofluorescence whereas superficial drusen appear bright, or hyper-autofluorescent. The Optic Disc Drusen Studies Consortium (ODDS) found that the majority of eyes with one superficial druse also had at least one buried druse.4 Deeply buried drusen are not visible with FAF, but B-scan ultrasonography can be used to detect buried drusen and is often indicated.5
Optic disc drusen are small, calcified deposits that become more apparent with age. They are typically buried during childhood and may initially appear as an optic disc with indistinct margins. With age, they gradually become more superficial and present with a bumpy appearance.
Optic disc drusen may predispose a patient to visual field defects, as seen in this patient, non-arteritic anterior ischemic optic neuropathy (NAION), subretinal hemorrhages and peripapillary choroidal neovascular membranes.6
Clinicians must also consider the possibility of a simultaneous presentation of disc edema and disc drusen; however, in this case overlying papilledema was considered less likely given the presence of a definite SVP and the absence of symptoms associated with increased intracranial pressure. Nonetheless, close serial monitoring of the disc appearance, afferent system and OCT is indicated to ensure no atypical progression.
 |
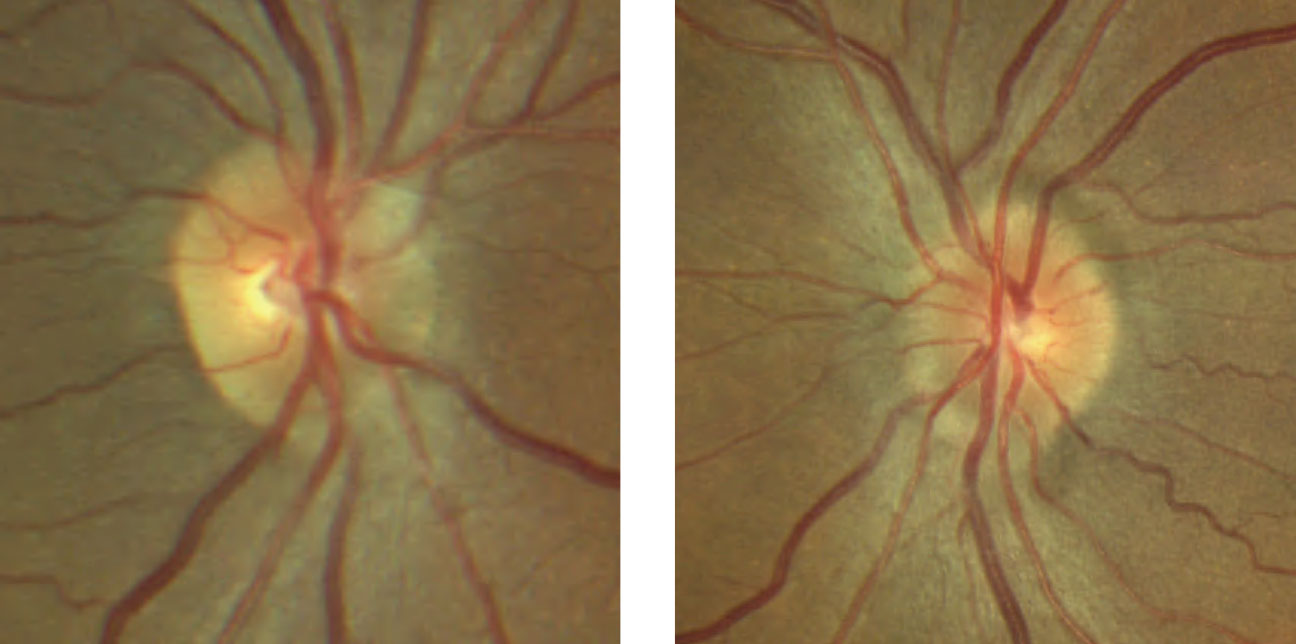
| Fig. 4. These color fundus photos of the optic discs show that the margins of the right optic disc, at left, are indistinct nasally but are otherwise preserved temporal. The left optic disc, at right, has more indistinct margins with a notable superficial druse superior nasal. Click image to enlarge. |
Case 3: Systemic Suspicion
A 76-year-old Caucasian male presented with a complaint of a progressively worsening “cloud” over his vision OS. He had seen another eye care provider approximately two months prior and the previous fundus photo demonstrated diffuse disc swelling OS. His ocular history was otherwise remarkable for a traumatic retinal detachment OD with resultant poor vision.
His medical history was remarkable for seropositive generalized myasthenia gravis. He was diagnosed four months prior and was treated with pyridostigmine as well as prednisone and intravenous immunoglobulin during exacerbations. His history was also significant for orthostatic hypotension.
His best-corrected visual acuity was counting fingers at one foot OD and 20/40- OS. The exam revealed a 3+ afferent pupillary defect OD. Confrontation visual fields were severely restricted OD and exhibited inferior constriction OS (Figure 6).
His anterior segment exam was unremarkable OS. Upon dilated fundus examination, the disc was flat and distinct OD, while the disc showed significant findings OS (Figure 7).
 |
| Fig. 5. The patient’s fundus autofluorescent photos demonstrate significant autofluorescence of the left optic disc, suggestive of more prominent drusen than what is evident funduscopically. Click image to enlarge. |
Discussion. The patient was diagnosed with sectoral disc edema in the left eye evidenced by blurred hyperemic disc margins inferiorly.
Note that there is no longer swelling of the superior neuroretinal rim as documented by previous examination, and it appears that he has subsequently developed pallor with a corresponding inferior visual field defect.
Potential differentials of unilateral disc edema include arteritic and non-arteritic AION and optic papillitis. Arteritic AION was considered less likely as the patient did not present with symptoms of giant cell arteritis (GCA) such as headache, jaw claudication, scalp tenderness, weight loss, reduced appetite, fatigue, amaurosis fugax or pallid disc swelling. However, given his age and the potential devastating consequences, testing to rule out GCA—in the form of serum platelet, ESR and CRP studies—was indicated and ordered.
Non-arteritic AION may be considered in this case given the patient’s age and history of orthostatic hypotension; however, given his monocular status and risk of further vision loss, laboratory workup to rule out any potential etiology of papillitis was indicated.
 |
| Fig. 6. The 24-2 automated visual field demonstrates an inferior defect OS. Click image to enlarge. |
Patients with papillitis often present with complaints of vision loss, and the examination will demonstrate a corresponding decrease in visual acuity, visual field loss and an afferent pupillary defect. Optic papillitis can be caused by inflammatory conditions, such as sarcoidosis, and infectious diseases, such as Lyme disease and syphilis.7
The patient was asked to complete laboratory testing that included ESR, CRP, FTA-ABS, RPR, ACE, Lyme titer and ANA.
If suspicion for sarcoidosis is high, consider ordering chest imaging, as ACE can be falsely low. Results were remarkable for elevated Lyme disease IgG and IgM antibodies on Western blot.
While causation may be difficult to establish with certainty here, it was important to identify his systemic Lyme infection, which could, if untreated, lead to further vision loss in a patient with already significant vision impairment.
 |
| Click table to enlarge. |
Case 4: Gaining Traction
A 52-year-old Caucasian male presented for evaluation of binocular vertical diplopia that began following a recent stroke. His medical history was remarkable for type II diabetes, hypertension, hypercholesterolemia, a right thalamic stroke, a myocardial infarction, asthma, sleep apnea, anxiety, depression and schizophrenia.
The patient’s best-corrected visual acuity was 20/20 OD and OS. There was no afferent pupillary defect and confrontation visual fields were normal, as was color vision.
Efferent testing demonstrated a vertical misalignment diagnosed as skew deviation attributed to his history of known right thalamic stroke. His dilated fundus exam was unremarkable in the right eye. His left eye showed significant changes in optic disc appearance and OCT imaging (Figure 8).
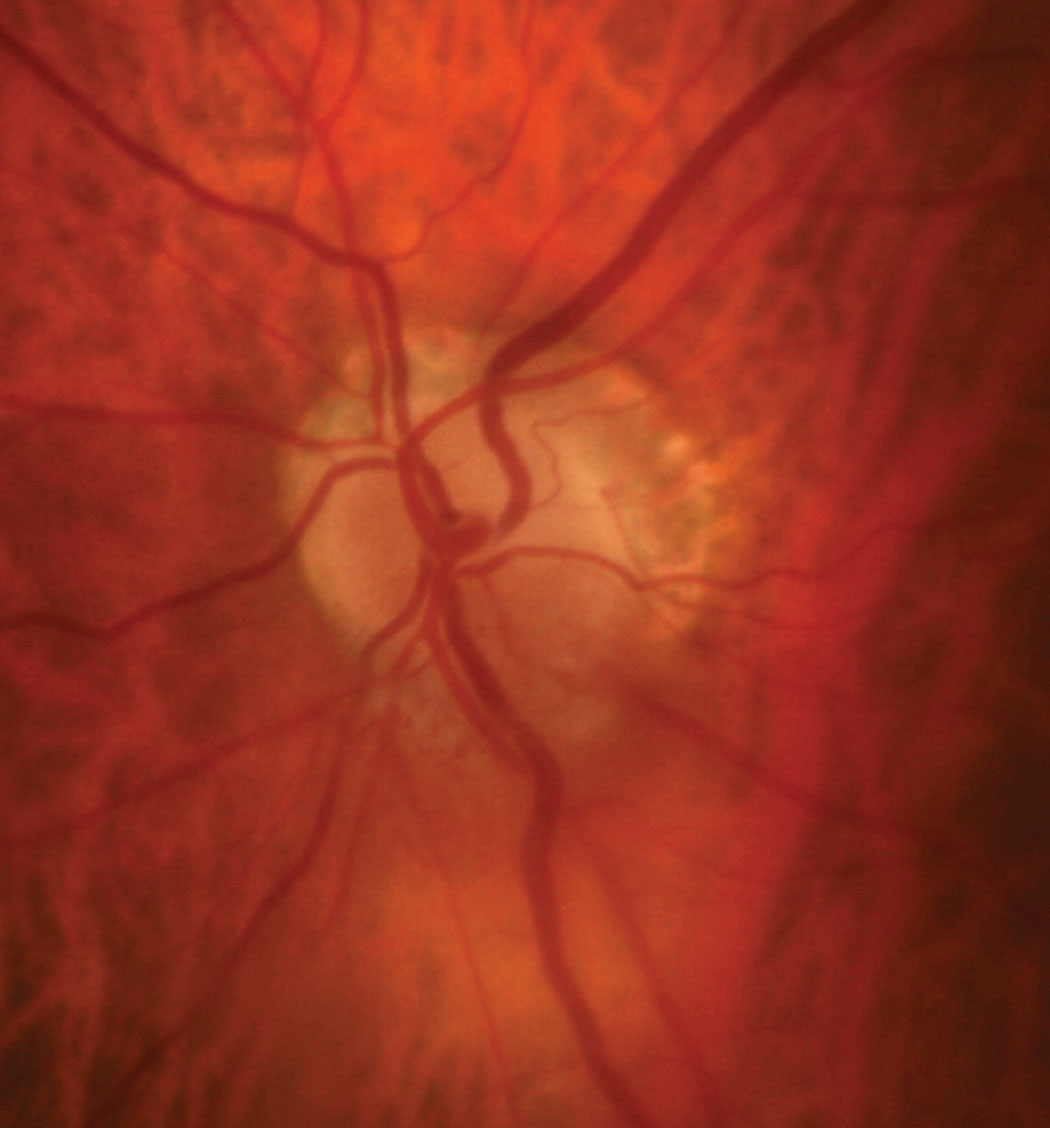 |
| Fig. 7. The patient’s optic disc photo demonstrates inferior sectoral elevation with hyperemia. Additionally, there is sectoral pallor of the superior neuroretinal rim OS. Click image to enlarge. |
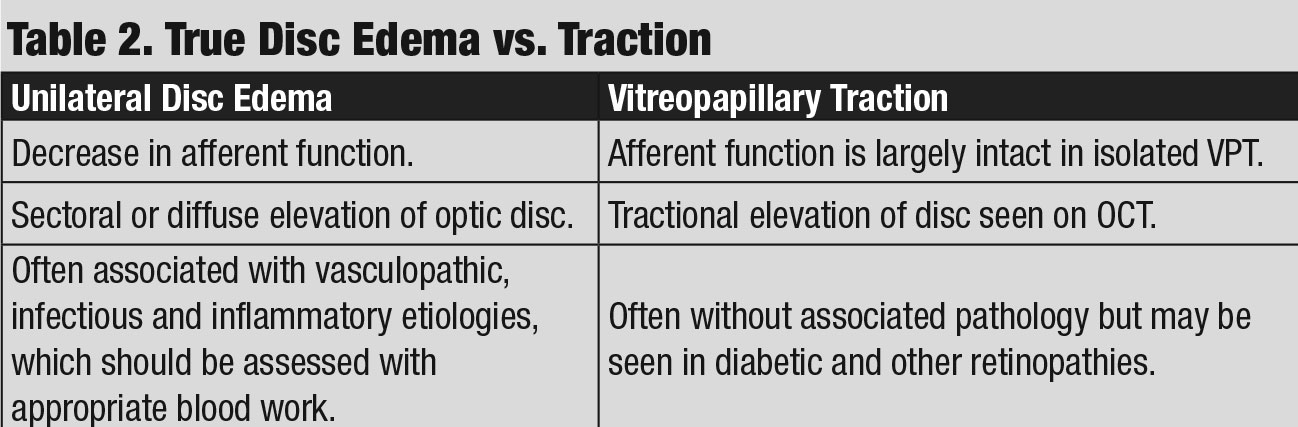
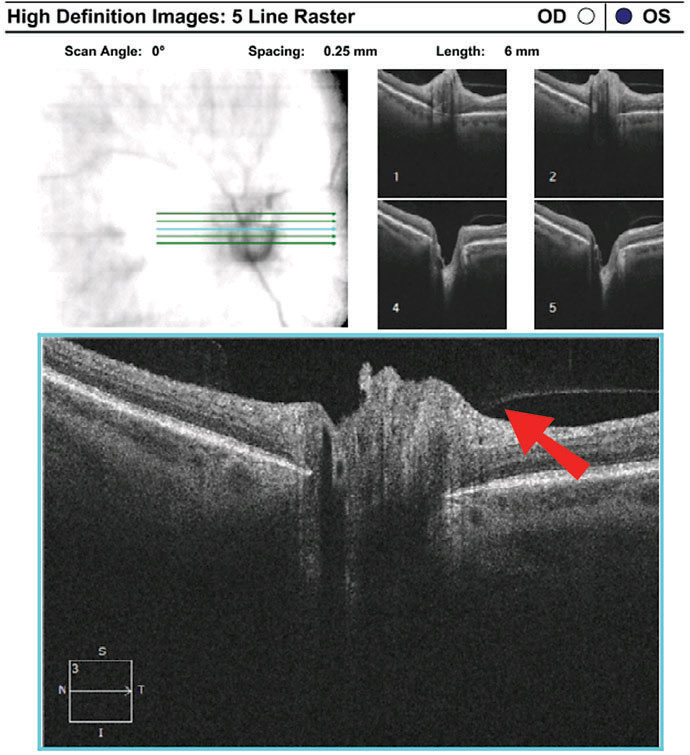
Discussion. As with case 3, this patient also exhibited sectoral disc elevation; however, the etiology is not true disc swelling but is instead tractional in nature. Vitreopapillary traction (VPT) is a condition caused by adherence of a fibrotic membrane or incomplete posterior vitreous detachment that raises the optic disc margin. This patient’s tractional elevation, induced by partial detachment of the posterior hyaloid face, can be visualized on the 5-line OCT raster.
In addition to elevation of the optic disc, VPT can result in indistinct optic disc margins and peripapillary hemorrhage, making it difficult to differentiate from true optic disc swelling, such as in AION and optic papillitis (Table 2).8
Therefore, clinicians must rule out these etiologies with serum lab testing. As such, CBC, ESR, CRP, ACE, ANA and RPR were ordered and unremarkable in this case. His negative blood work results, along with normal afferent function, helped to support the diagnosis of VPT as afferent visual function is often affected in cases of AION and optic papillitis.9
VPT has been described in both eyes without ocular pathology, as well as in eyes with pathology that may result in fibrotic membrane proliferation such as diabetic retinopathy and vein occlusion. Given the presence of telangiectatic vessels on the optic disc and systemic history, concurrent diabetic papillopathy could be considered in this case; but ultimately, long-term follow-up was helpful in excluding this diagnosis.10,11
This patient’s case demonstrates the importance of considering VPT in optic disc elevation and looking closely at the vitreoretinal interface on OCT.
 |
 |
| Fig. 8. Imaging reveals superior neuroretinal rim elevation with telangeictatic vessels. The OCT 5-line raster, top, demonstrates vitreopapillary adhesion. Click images to enlarge. |
Case 5: A Pale Masquerader
A 15-year-old African American male presented for re-evaluation of his optic disc OS. He was seen one year prior by another provider and was diagnosed with refractive amblyopia OD and suspected pseudo-temporal pallor OS. He denied any visual complaints or changes. He denied any history of trauma or neurologic symptoms such as headaches. His medical history was remarkable for asthma.
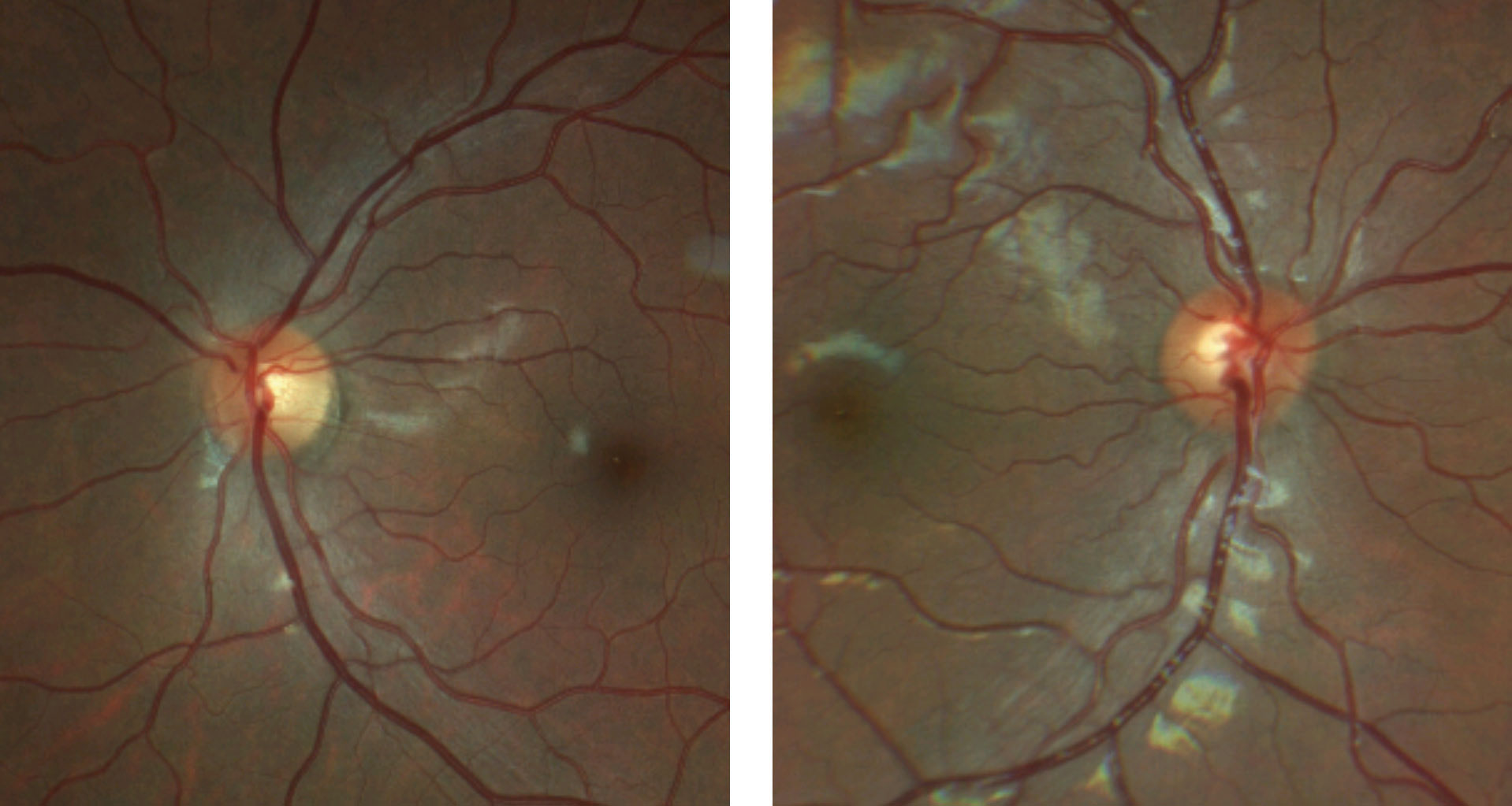
His best-corrected visual acuity was stable at 20/40 OD, 20/20 OS. Pupils were equal, round and reactive to light with no afferent pupillary defect OS. Confrontation and automated visual fields were full without defects OU and color vision was normal. Refraction was remarkable for amblyogenic hyperopia OD. His posterior segment exam and OCT measurements were repeated and compared to findings from one year prior, with significant findings (Figures 9 and 10).
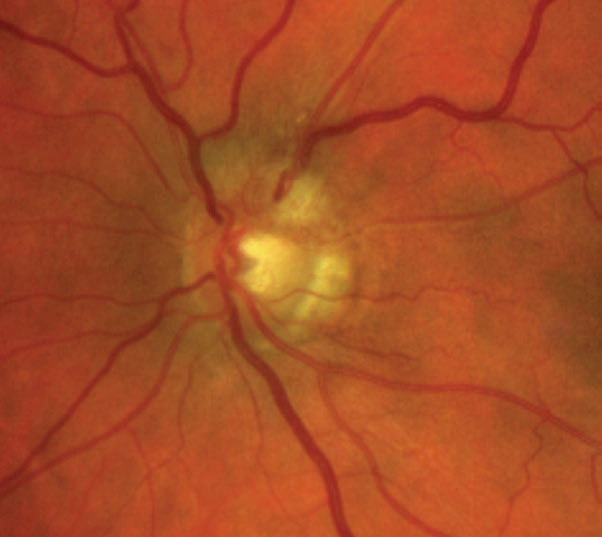
 |
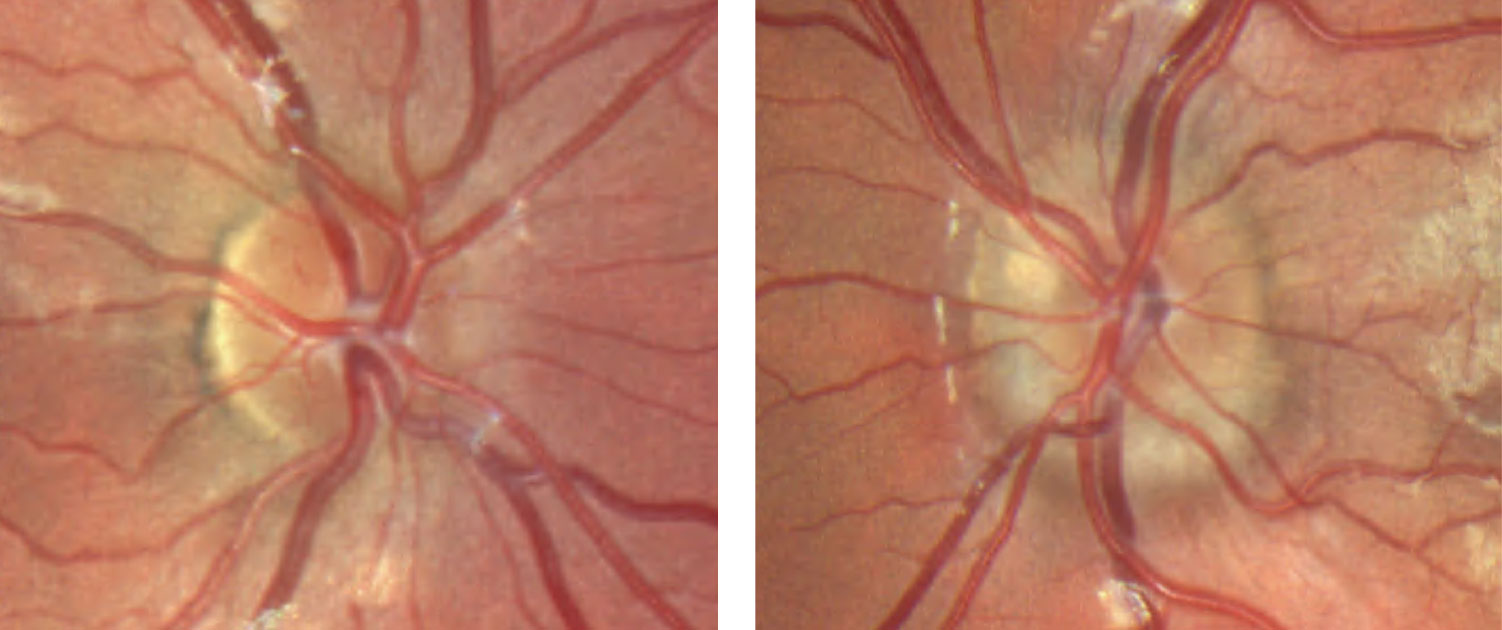
| Fig. 9. This patient’s fundus photo suggests a pale temporal neuroretinal rim in the left eye. Click image to enlarge. |
Discussion. The diagnosis of stable pseudo-temporal pallor OS was made based on normal afferent function in the left eye and OCT.
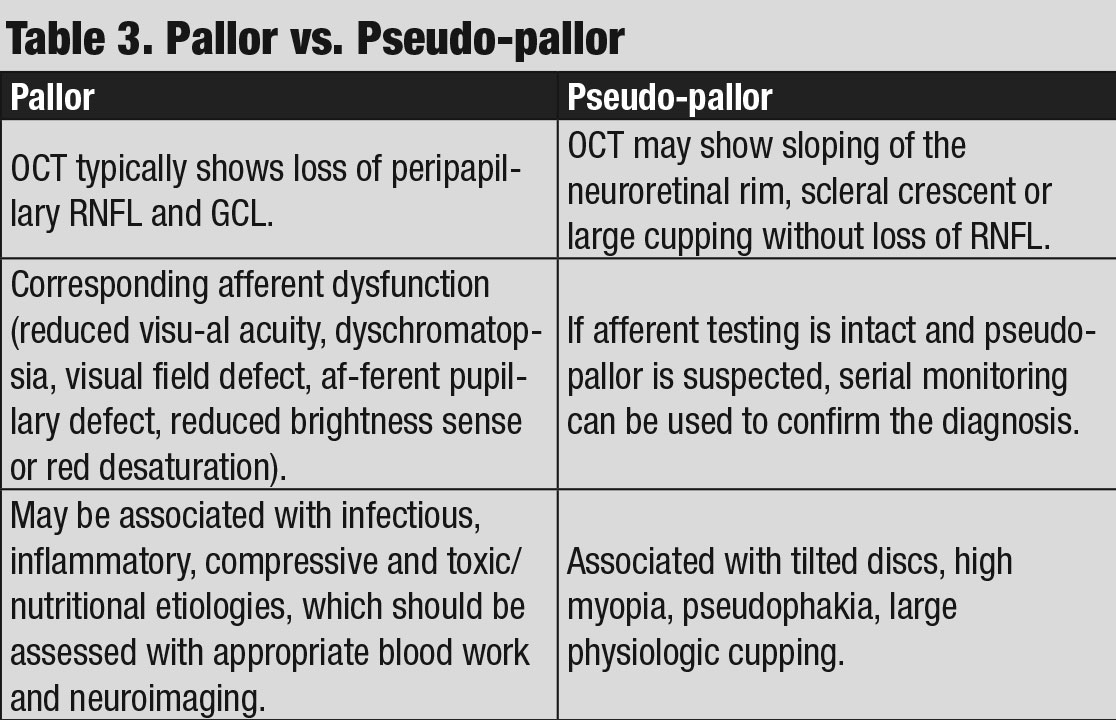
While some anomalous discs may exhibit associated afferent findings, the lack of any abnormalities OS was significant in this case, as true pallor is typically associated with afferent pupillary defect, color vision loss, visual field defect or a combination of all three (Table 3).
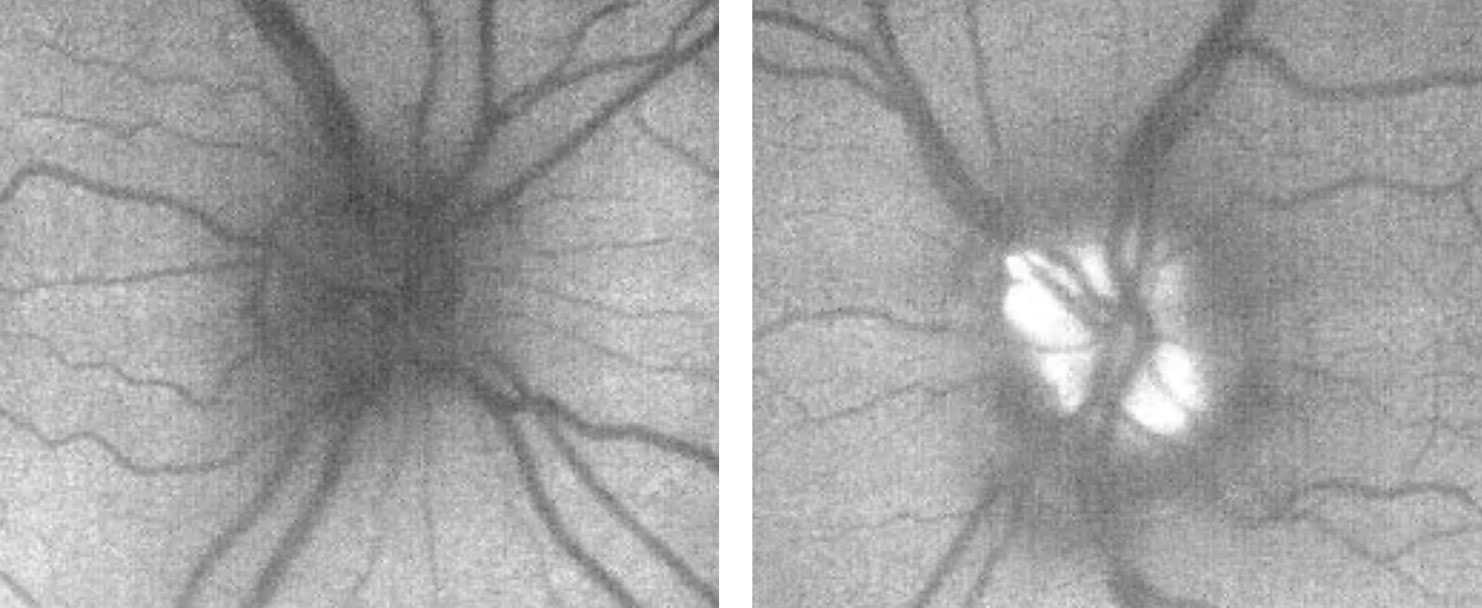
OCT of the optic disc and peripapillary retinal nerve fiber layer (RNFL) is a valuable tool to look for anatomic variations or anomalies that can give rise to the appearance of pseudo-pallor. For example, note in the patient’s OCT the asymmetric disc diameter with the left disc (the one in question) being notably smaller than the right. This is important in this case, as small/hypoplastic discs may appear pale temporally, especially if there is a concurrent scleral crescent
Additionally, tilted optic discs can have a similar appearance and, in this case, a subtle tilt of the disc can be appreciated by viewing the horizontal tomogram on the OCT in which the temporal neuroretinal rim is lower and sloped.
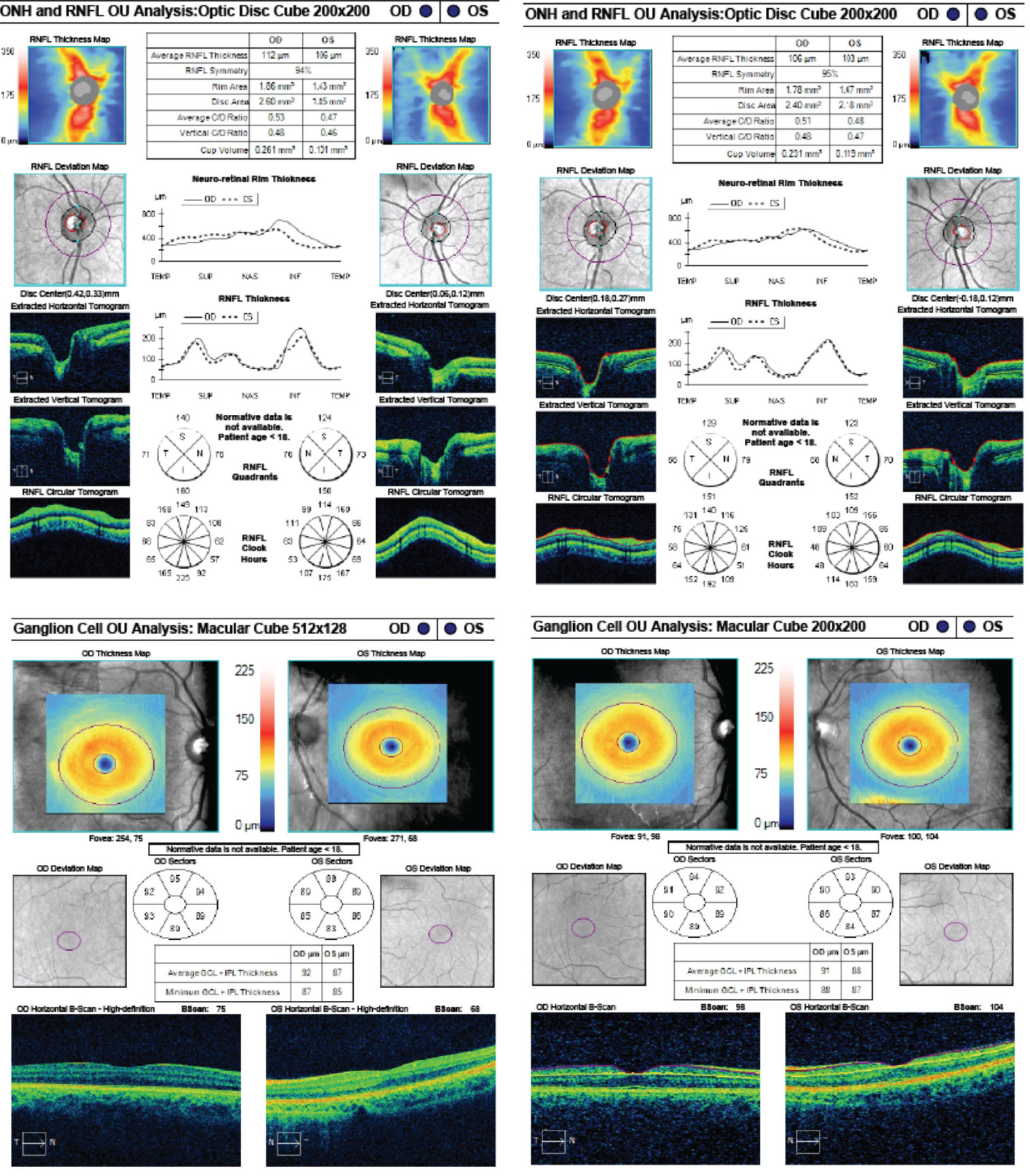 |
| Fig. 10. These are the patient’s OCT images upon initial exam, at left, and one year later, at right. The OCT demonstrates an intact RNFL and GCL in both eyes. Note the small disc area OS compared with OD, as well as gradual sloping of the temporal aspect of the cup OS compared with the symmetric margins of the cup OD. Click image to enlarge. |
However, interpreting OCT of the peripapillary RNFL for thinning can be complicated by anatomical variation, such as shifted RNFL bundles, or even pathology, such as disc swelling. Ganglion cell layer (GCL) analysis, in contrast, may not be as affected by anatomical difference/swelling and can be a valuable adjunctive tool for detecting retinal ganglion cell death, implicating an optic neuropathy.12,13 Therefore, GCL analysis was beneficial as this ruled out any thinning or loss suggestive of an optic neuropathy.
Other conditions that a clinician may be confronted with that can mimic pallor include pseudophakic pallor, which is caused by change in the lens optics, and large physiologic cupping.14 In addition to a thorough afferent evaluation and RNFL/GCL OCT, repeat evaluation to ensure stability is helpful in confirming pseudo-pallor, as opposed to pallor caused by an active process.
Ultimately, while these tools can help differentiate true pallor from pseudo-pallor, if the judgement cannot be made with confidence, further work-up to rule out potentially treatable causes of optic neuropathy may be indicated.
Careful clinical examination in conjunction with ancillary testing such as OCT and visual fields are important in differentiating benign processes from potential neuro-ophthalmologic emergencies. Critical assessment of the peripapillary region and optic nerve head for neuroretinal rim thinning, pallor and elevation is important in all patients to identify subtle disc anomalies and make the correct diagnosis.
Dr. Maglione works in the neuro-ophthalmic disease services at The Eye Institute and teaches didactically in neuro-anatomy and neuro-ophthlamic disease courses at the Pennsylvania College of Optometry at Salus University.
Dr. Seidler graduated from the Pennsylvania College of Optometry at Salus University and recently completed a two-year advanced residency program at The Eye Institute in neuro-ophthalmic disease.
The authors would like to thank Erin Draper, OD, and Kelly Malloy, OD, for their mentorship and guidance.
| 1. Hingwala DR, Kesavadas C, Thomas B, et al. Imaging signs in idiopathic intracranial hypertension: Are these signs seen in secondary intracranial hypertension too?. Ann Indian Acad Neurol. 2013;16(2):229-33. 2. Alnemari A, Mansour TR, Gregory S, et al. Chiari I malformation with underlying pseudotumor cerebri: Poor symptom relief following posterior decompression surgery. Int J Surg Case Rep. 2017;38:136-41. 3. Aiken AH, Hoots JA, Saindane AM, Hudgins PA. Incidence of cerebellar tonsillar ectopia in idiopathic intracranial hypertension: a mimic of the Chiari I malformation. AJNR Am J Neuroradiol. 2012;33(10):1901-06. 4. Malmqvist L, Bursztyn L, Costello F, et al. The Optic Disc Drusen Studies Consortium recommendations for diagnosis of optic disc drusen using optical coherence tomography, J Neuro-Ophthalmol. 2018;38(3):299-307. 5. Tugcu B, Özdemir H. Imaging methods in the diagnosis of optic disc drusen. Turk J Ophthalmol. 2016;46(5):232-36. 6. Palmer E, Gale J, Crowston JG, Wells AP. Optic nerve head drusen: an update. Neuro-Ophthalmol. 2018;42(6):367-84. 7. Kahloun R, Abroug N, Ksiaa I, et al. Infectious optic neuropathies: a clinical update. Eye Brain. 2015;7:59-81. 8. Hedges TR, Flattem NL, Bagga A. Vitreopapillary traction confirmed by optical coherence tomography. Arch Ophthalmol. 2006;124(2):279-81. 9. Gabriel RS, Boisvert CJ, Mehta MC. Review of vitreopapillary traction syndrome. Neuro-ophthalmol. February 26, 202. [Epub ahead of print]. 10. Regillo CD, Brown GC, Savino PJ, et al. Diabetic papillopathy: patient characteristics and fundus findings. Arch Ophthalmol. 1995;113(7):889-95. 11. Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92-108. 12. Chen JJ, Kardon RH. Avoiding clinical misinterpretation and artifacts of optical coherence tomography analysis of the optic nerve, retinal nerve fiber layer, and ganglion cell layer. J Neuro-ophthalmol. 2016;36(4):417-38. 13. Vieira LMC, Silva NFA, Dias dos Santos AM, et al. Retinal ganglion cell layer analysis by optical coherence tomography in toxic and nutritional optic neuropathy. J Neuro-ophthalmol. 2015;35(3):242-45. 14. Digre KB, Corbett JJ. Is the disc pale? In: Practical Viewing of the Optic Disc. Amsterdam: Butterworth-Heinemann; 2003:193-200. |

