Optical coherence tomography (OCT) has had a transformative impact on eye care. From detecting retinal fluid and preserving sight to monitoring glaucoma and maintaining vision, OCT has become an indispensable tool for posterior segment disease management. One of its newest iterations, anterior segment OCT (AS-OCT), is proving to be valuable for a wide variety of anterior segment applications and is becoming an integral part of specialty contact lens prescribing.
Whether they help restore sight for patients with keratoconus or relieve severe ocular discomfort for patients with dry eye disease (DED), specialty contact lenses can be life changing. However, fitting can be time-consuming and imprecise, as clinicians may need to rely on trial and error. This is where AS-OCT comes into play. Here is a comprehensive look at how AS-OCT can improve precision and proficiency in specialty contact lens eye care.
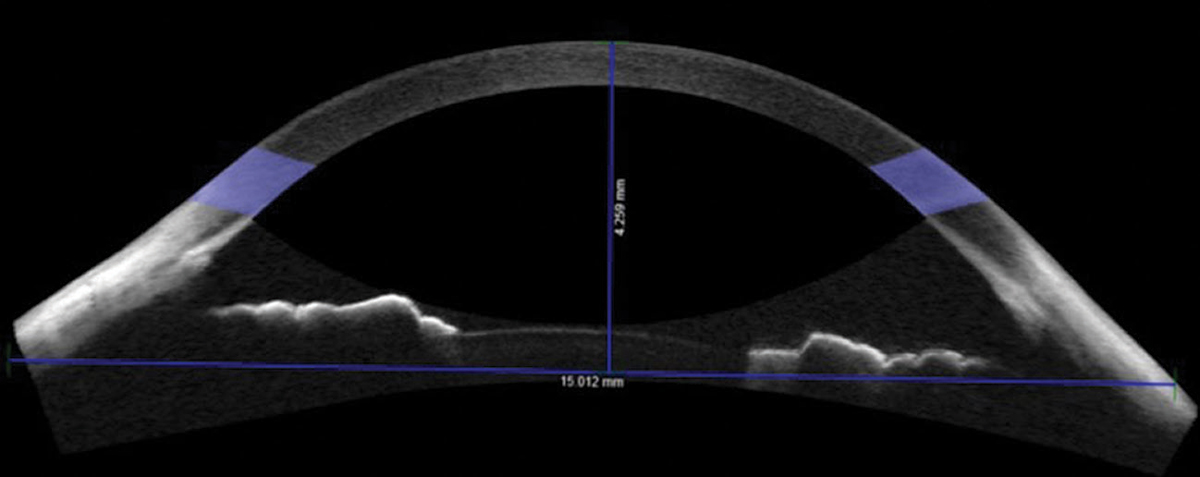 |
| Fig. 1. Non-custom soft contact lenses didn’t fit right for this patient with a sagittal height of 4,259µm. Consider custom lenses in these cases. Click image to enlarge. |
Precise, Accurate Measurements
AS-OCT offers a wide array of measurements that can benefit specialty lens fitting and safeguard ocular health. The technology can provide information on the anterior eye that is particularly useful in refining specialty lens prescriptions.
Sagittal height and depth. While clinicians had to estimate corneal-scleral sagittal height in the past, AS-OCT can now aid in selecting the optimal scleral lens sagittal depth. AS-OCT provides precise measurements that can increase initial lens success.1,2
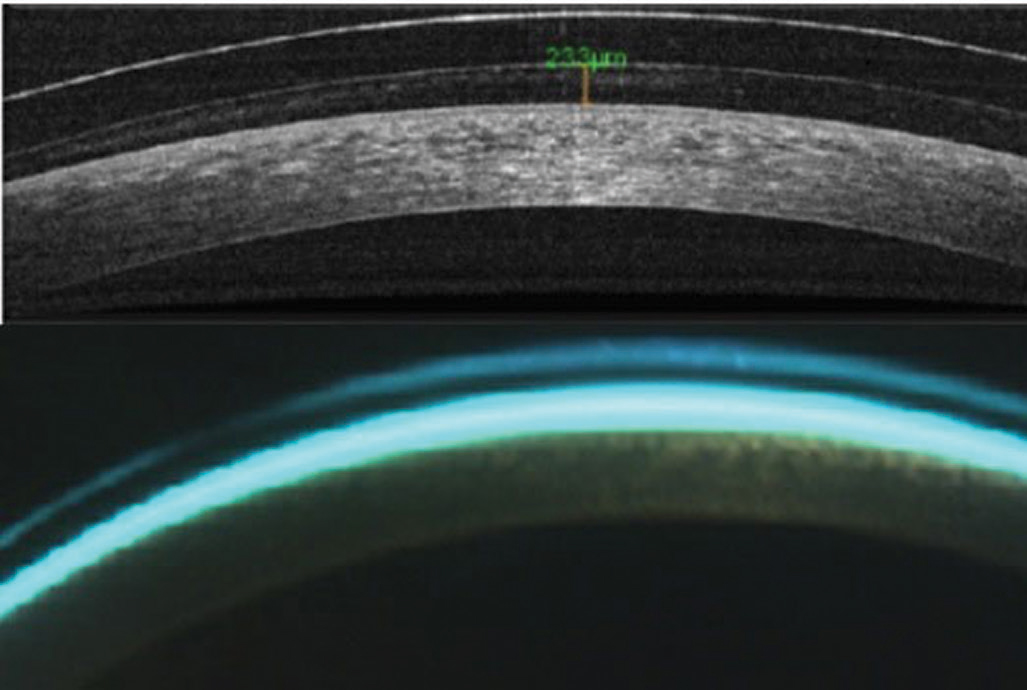 |
| Fig. 2. AS-OCT (top) and biomicroscopy of central scleral lens clearance. Click image to enlarge. |
For a lens close to 15mm in diameter, the fitter can use AS-OCT to measure the sagittal height of the anterior segment at the 15mm cord and add it to the desired initial central lens clearance. For example, if the sagittal height of the anterior segment at the 15mm chord is 4,000µm and the desired initial central lens clearance is 300µm, combining the two to find a sagittal lens depth of 4,300µm is a good starting point. The 15mm chord measurement also shows pupil size and angle kappa, which may be useful in designing multifocal lenses.
A larger sagittal depth is required for lenses larger than 15mm. Based on literature and experience, an adjustment of 400µm per each 1mm change in lens diameter, such that 4,700µm is the ideal initial sagittal depth for a 16mm diameter lens in the previous patient’s case, is usually reliable.3-6
Conducting AS-OCT chord measurements is valuable beyond scleral lens selection. It is also beneficial for soft lens selection. At the 15mm chord, the mean sagittal height of normal eyes is approximately 3,800µm.3-5 Patients with sagittal height measurements less than 3,500µm or greater than 4,100µm may require a custom soft contact lens or a specialized fitting approach (Figure 1).3-5
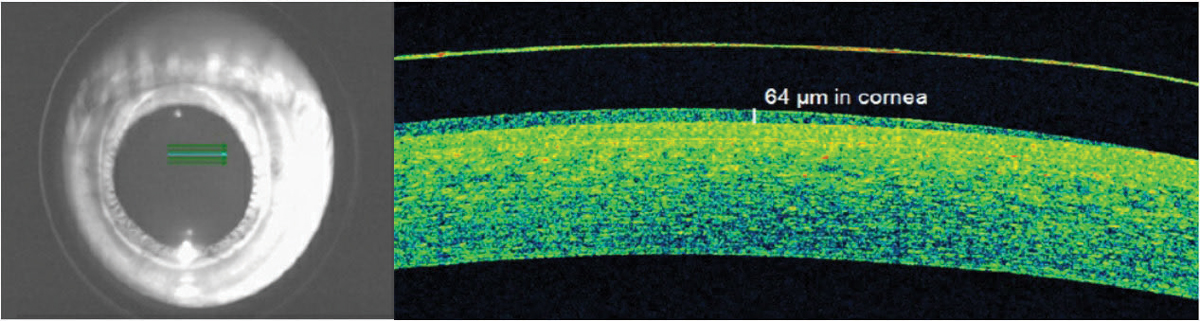 |
| Fig. 3. OCT identified acceptable apical hybrid lens clearance on a keratoconus patient when slit lamp estimates were variable. Click image to enlarge. |
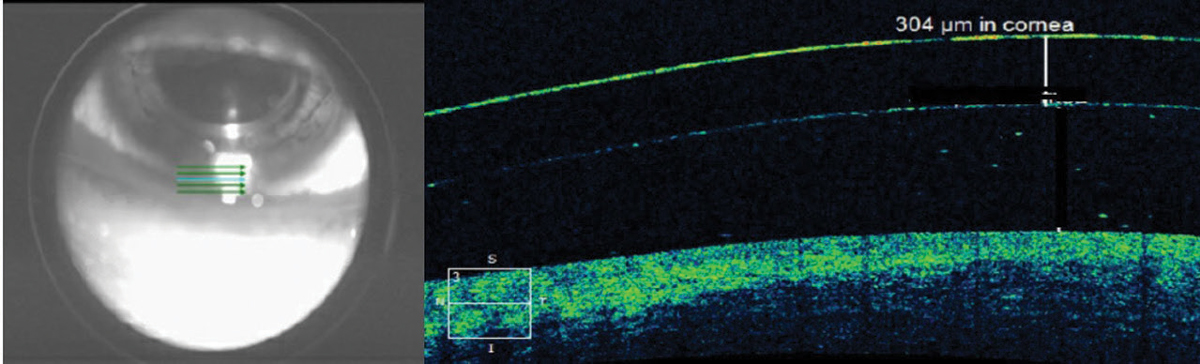 |
| Fig. 4. The scleral lens thickness at the inferior limbus is thicker than the manufacturer-listed center thickness for this low-powered lens. Click image to enlarge. |
Tear layer lens clearance. Posterior lens tear layer thickness is important when fitting scleral and hybrid lenses. AS-OCT can improve measurement accuracy, considering the clinically significant overestimation of central tear layer lens clearances of scleral lenses with slit lamps (Figure 2).7-9 Central clearances for scleral and hybrid lenses designed to vault the cornea should be no lower than 100µm before lens settling and 50µm after to prevent corneal bearing (Figure 3).10-13
AS-OCT scleral lens limbal clearance measurements are also critical for a proper fit, especially as biomicroscopic assessments of limbal clearance can be challenging. While a well-fit scleral lens should clear the limbus by 10µm to 60µm, a fluorescein-stained tear layer doesn’t become visible until 20µm to 30µm.14-19 Consequently, a clinician may erroneously believe a well-fit scleral lens has limbal bearing.
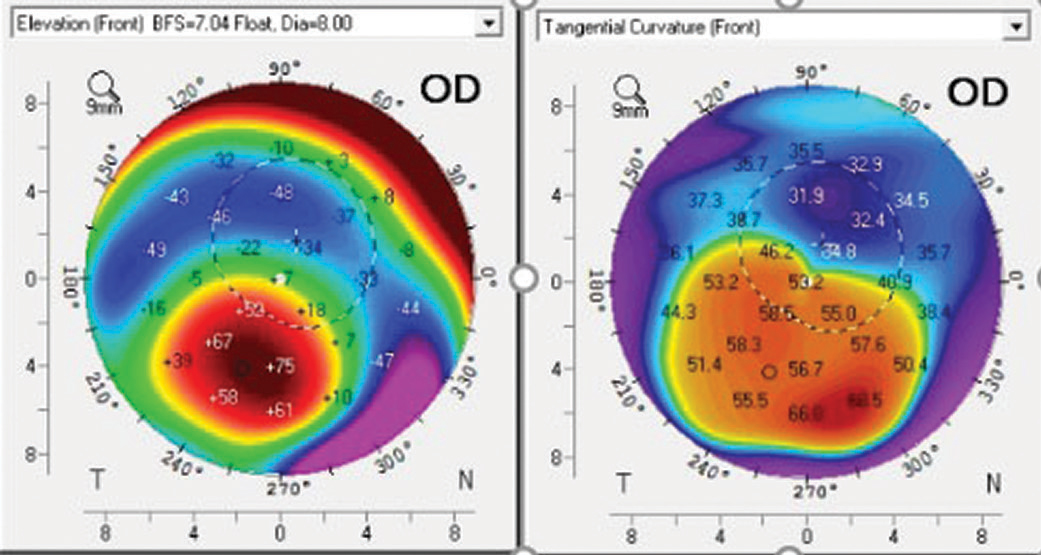 |
| Fig. 5. Corneal topography of a post-PK patient shows the corneal apex is outside the center of the cornea. Click image to enlarge. |
Inherent inaccuracies in the subjective estimation of limbal clearance put AS-OCT measurements on an even higher pedestal. With subjective estimation, clinicians compare the post-lens tear layer thickness with the manufacturer’s published center thickness. However, lens thickness varies significantly across the lens and with back vertex power (Figure 4).20,21AS-OCT measurements are valuable when lens clearance must be assessed outside of the center of the lens, such as when the limbus is concerned or when the apex of an irregular cornea lies outside of the center of the lens in cases of keratoconus, pellucid marginal degeneration and post-graft patients (Figure 5). As with central and limbal areas of the cornea, excessive lens clearance or bearing is concerning at the corneal apex, a complication that AS-OCT may prevent.11
While advantageous, obtaining lens clearance measurements from multiple locations can be time-consuming. Fortunately, some commercial OCT instruments now generate color-coded corneal clearance maps to offer more rapid assessments of corneal clearance.21
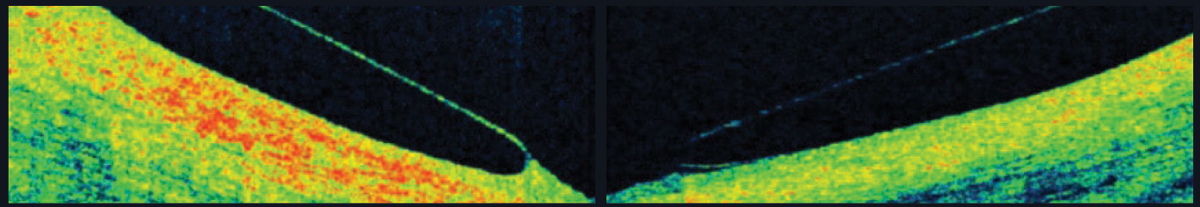 |
| Fig. 6. AS-OCT shows a scleral lens edge with slight impingement into the conjunctiva (left) and an edge that lifts away from the conjunctiva (right). |
Thickness and alignment. Lens thickness variance across scleral, corneal gas permeable (GP) and custom soft lenses is particularly evident with high back surface powers. Oxygen transmissibility across the surface of specialty lenses is important, so lens thickness is a crucial measurement, especially in cases of high powers. Lens thickness modification or lens wear reduction may prevent complications associated with hypoxia.
Landing zone. Scleral lenses land on the conjunctiva in a way that the lens weight should distribute evenly and the lens should neither sink into nor lift off of the conjunctiva. If the lens sinks, it could cause subtle, unwanted vessel constriction; if it rises, signs of discomfort not visible by slip lamp can be seen on OCT (Figure 6).
Get to the Root of the Problem
Comfort, vision and health are essential aspects of success with contact lenses. But specialty lenses involve more than just designing lenses that can restore or enhance vision. By the nature of the conditions that require these lenses, fitters are frequently charged with unmasking and monitoring various anterior segment conditions. AS-OCT can shed light on the causes of a suboptimal wearing experience when biomicroscopy detection fails and characterizing a variety of corneal dystrophies and degenerations (Figure 7).22,23 This includes delineating pathognomonic features and deposits within corneal sub-layers and monitoring corneal changes with contact lens wear.22,23
Discomfort. A defective lens edge on any design can cause lens discomfort. Determining if a poor-quality lens edge is the source of discomfort can be elusive with biomicroscopy. AS-OCT imaging of a lens landing zone can easily uncover if a defective lens edge is the culprit.
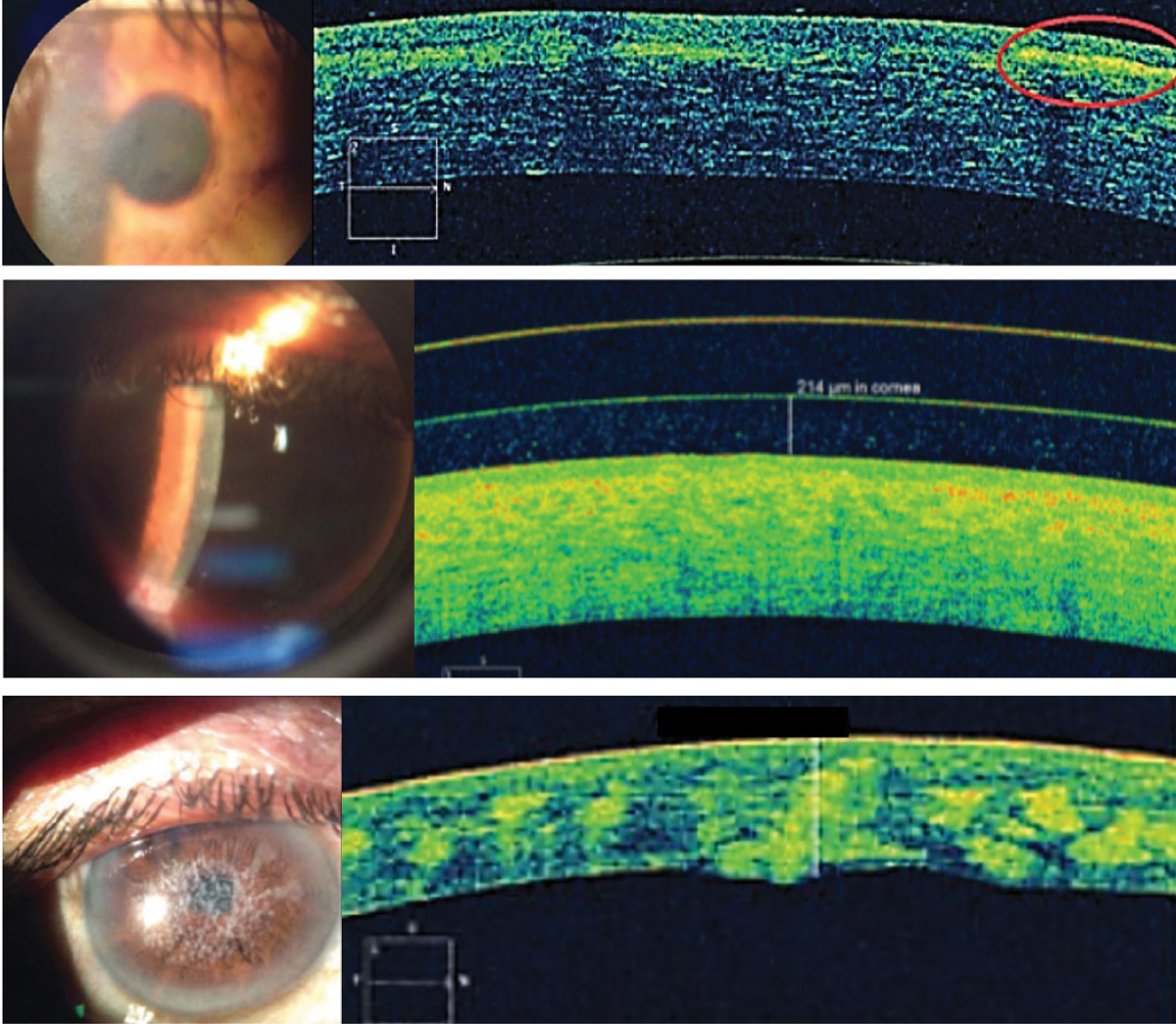 |
| Fig. 7. From the top, Reis-Bücklers corneal dystrophy, lattice corneal dystrophy and type 1 granular corneal dystrophy with hyper-reflective deposits at Bowman’s layer, in the anterior stroma and in the stroma, respectively. Click image to enlarge. |
Blur. Visual blur with specialty lens wear can occur due to debris accumulation underneath the lens. Post-lens tear layer debris accumulation is common in scleral lens wear and creates a fog-like blur in 20% to 33% of patients (Figure 8).17,21,24 AS-OCT imaging enables practitioners to observe visually impacting post-lens tear layer debris that is not always detectable at the slit lamp.
Corneal edema. Lens wear can induce corneal edema, which is especially challenging when fitting corneas prone to edema, such as post-penetrating keratoplasty and Fuchs’ dystrophy and others with low endothelial cell counts. Patients should have no more than 5% of edema (Figure 9).25
Commercial OCT instruments now offer corneal thickness and pachymetry mapping to allow for efficient discovery and monitoring of edema that may be undetectable at the slit lamp. Additionally, this tool permits non-contact corneal thickness assessment with contact lens wear when conventional techniques cannot provide accurate measurements. OCT monitoring of corneal swelling in patients prone to edema is invaluable for safeguarding long-term ocular health and successful contact lens outcomes.
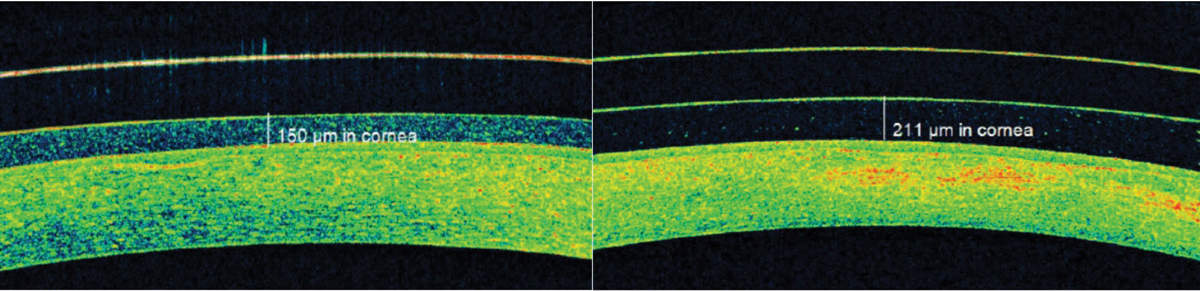 |
| Fig. 8. AS-OCT identified significant post-scleral lens tear layer debris that was causing subtle but bothersome foggy vision. Click image to enlarge. |
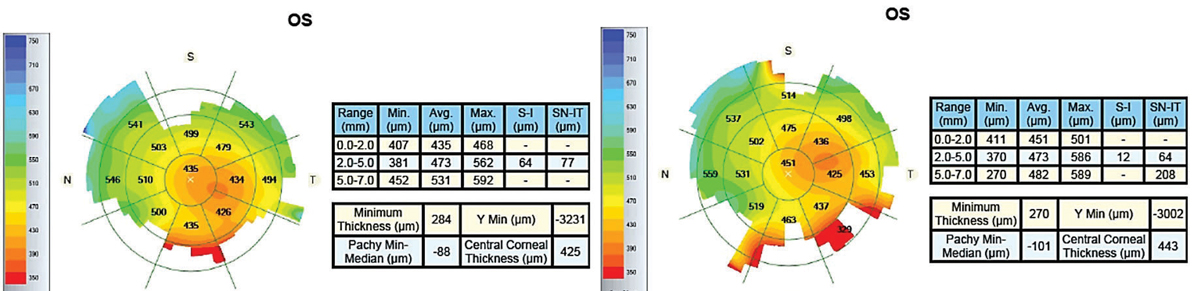 |
| Fig. 9. Pre-scleral lens wear (left) and post-two hours of lens wear imaging (right) of an advanced keratoconus patient with a history of acute hydrops and pathological edema indicated central corneal swelling. |
Keratoconus. Detecting keratoconus is crucial in preventing corneal ectasia secondary to LASIK.26 Early detection helps delay corneal transplantation with corneal collagen crosslinking. However, the early stages of keratoconus are not easy to diagnose with conventional techniques because these eyes frequently have normal clinical findings that do not stand out on topography.26-29 This is where OCT corneal epithelial mapping can be useful.26,30,31
Research shows that epithelial thickness in the thinnest corneal zone can diagnose forme fruste keratoconus.27 In keratoconus, the locations of epithelial thinning on OCT thickness maps are also usually inferotemporal and roughly consistent with the location of corneal steepening shown on a corneal topography map.32 While studies have investigated epithelial thickness patterns characteristic of keratoconus, keratoconus diagnosis still requires a clinician to recognize patterns on OCT pachymetry and epithelial thickness maps and correlate them with corneal topography and clinical information.32-35
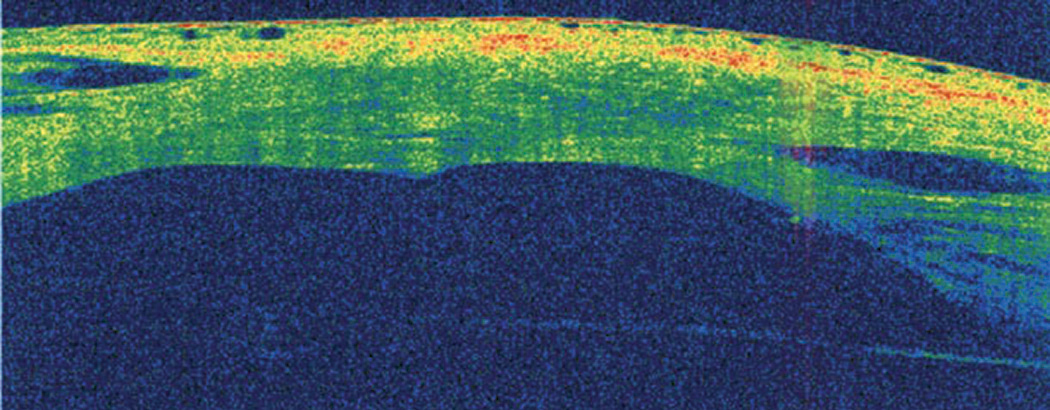 |
| Fig. 10. AS-OCT is useful for monitoring the healing process of acute corneal hydrops. Click image to enlarge. |
AS-OCT may also help identify anatomic features predictive of acute corneal hydrops and future penetrating keratoplasty.23,36 In a study involving eyes with advanced keratoconus, increased epithelial thinning, stromal thinning at the cone, anterior hyper-reflective areas in Bowman’s layer and the absence of stromal scarring on AS-OCT were potential predictive factors for the development of acute corneal hydrops.23,36 AS-OCT is also very valuable in monitoring the healing process of acute hydrops (Figure 10).
Inflammation and infection. While contact lenses are an effective form of vision correction, wearing them increases an individual’s risk for sight-threatening infections and associated inflammation.37 AS-OCT can be used to monitor the treatment of contact lens-associated infiltrative events. Objectively monitoring treatment response involves assessing the degree of regression of a stromal hyper-reflective band indicating infiltration of the corneal stroma and changes to corneal thickness.23,38
Anterior chamber cellular reaction is key in establishing inflammation severity and treatment effectiveness. AS-OCT outperforms slit lamp evaluation by enabling automated anterior chamber cell grading (for consistent inter- and intra-clinician evaluation) and the evaluation of anterior chamber inflammation in corneas with impaired clarity, such as scarred eyes with advanced keratoconus (Figure 11).23,39
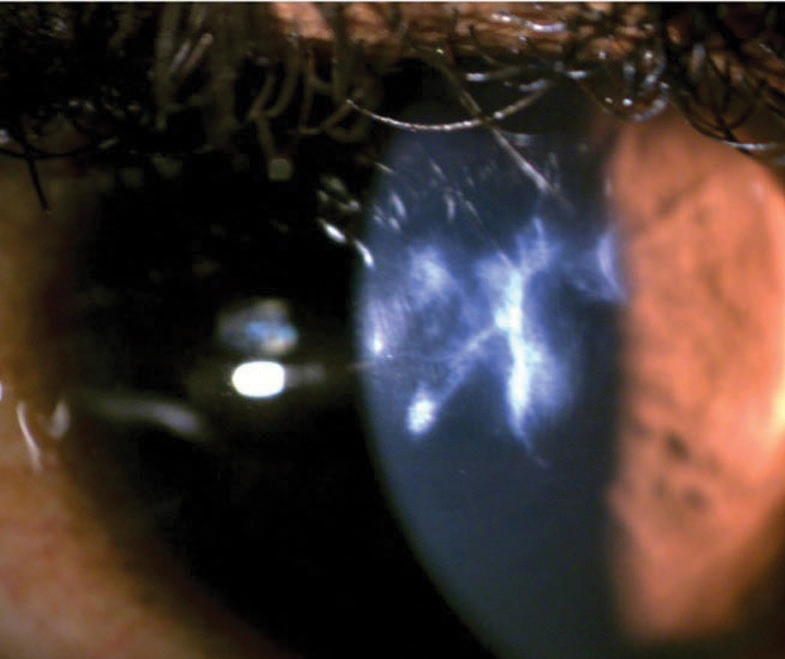 |
| Fig. 11. Accurately assessing the anterior chamber reaction with a slit lamp would be challenging in this case of severe keratoconus with a scarred cornea. Click image to enlarge. |
Dry eye. Dry eye is common in contact lens wearers, with at least 40% of users reporting dry eye symptoms.40 Conventional dry eye diagnostic tests, such as Schirmer’s tear-wetting and ocular surface dye-staining, are invasive and subjective, which can negatively influence result accuracy. To prevent this, objective assessments of the tear film are needed.41,42 AS-OCT can more precisely and objectively quantify markers of DED, such as tear film layer thickness and tear meniscus height, than conventional means of a slit lamp beam.43,44 With AS-OCT, the highly reflective tear film is more distinguishable from the cornea.45 For tear meniscus height measurements, clinicians can place the AS-OCT crosshair at the lid margin and use the caliper to measure between the fornix of the globe and the palpebral conjunctiva.
While AS-OCT tear film measurements have a moderate correlation with the degree of dry eye symptoms, they are very useful for monitoring a patient’s response to dry eye treatments, such as artificial tears and punctal occlusion.46-49 AS-OCT corneal epithelial mapping can also aid in DED detection, as irregular epithelium thickness patterns are observed in dry eye.50 In the future, OCT may become more of a simple, patient-friendly method for obtaining information on meibomian gland microscopic structural changes.51 AS-OCT’s objective and efficient role in DED identification and monitoring warrants its involvement in diagnostic protocols to improve outcomes.
OCT imaging offers far more than what the technology set out to do at its inception 30 years ago. It has advanced specialty lens practice by refining lens selection, quantifying fit aspects and troubleshooting related concerns. OCT has helped clinicians restore, enhance and maintain sight for many patients, who in turn are now able to visualize undesirable contact lens complications and better understand their corneal state and efficient wear and care techniques. OCT’s role in educating patients on their condition and improving compliance with treatment regimens cannot be overstated. Here, a picture is truly worth a thousand words.
Dr. Mickles is an associate professor at the Nova Southeastern University College of Optometry, the coordinator of the Dry Eye Care Center and a fellow of the American Academy of Optometry and the Scleral Lens Education Society.
| 1. Sorbara L, Maram J, Fonn D, et al. Metrics of the normal cornea: anterior segment imaging with the Visante OCT. Clin Exp Optom. 2010;93(3):150-6. 2. Gemoules G. A novel method of fitting scleral lenses using high resolution optical coherence tomography. Eye Contact Lens. 2008;34(2):80-3. 3. Kojima R, Caroline P, Graff T, et al. Eye shape and scleral lenses. Contact Lens Spec. 2013;28 38-43. 4. Copilevitz L. Are you scared of heights? Poster presented at the Global Specialty Lens Symposium. January 27, 2012; Las Vegas, NV. 5. Achong-Coan R. How do normal and keratoconic eyes differ in shape? Poster presented at the Global Specialty Lens Symposium. January 27, 2012; Las Vegas, NV. 6. Jedlicka J. Critical measurements to improve scleral lens fitting. Rev Cornea Contact Lenses. 2015;9:26-31. 7. Brujic M. Estimating scleral lens clearance and comparing it to OCT measured clearance. Poster presented at the Global Specialty Lens Symposium. January 22, 2015; Las Vegas, NV. 8. Fuller DG, Chan N, Smith B. Neophyte skill judging corneoscleral lens clearance. Optom Vis Sci. 2016;93(3):300-4. 9. Yeung D, Sorbara L. Scleral lens clearance assessment with biomicroscopy and anterior segment optical coherence tomography. Optom Vis Sci. 2018;95(1):13-20. 10. Vincent SJ, Alonso-Caneiro D, Collins MJ. The temporal dynamics of miniscleral contact lenses: central corneal clearance and centration. Cont Lens Anterior Eye. 2018;41(2):162-8. 11. Esen F, Toker E. Influence of apical clearance on mini-scleral lens settling, clinical performance, and corneal thickness changes. Eye Contact Lens. 2017;43(4):230-5. 12. Kauffman MJ, Gilmartin CA, Bennett ES, et al. A comparison of the short-term settling of three scleral lens designs. Optom Vis Sci. 2014;91(12):1462-6. 13. SynergEyes. UltraHealth Contact Lens Fitting Guide. 14. Michaud L, van der Worp E, Brazeau D, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Anterior Eye. 2012;35(6):266-71. 15. Jaynes JM, Edrington TB, Weissman BA. Predicting scleral GP lens entrapped tear layer oxygen tensions. Cont Lens Anterior Eye. 2015;38(1):44-7. 16. Compañ V, Aguilella-Arzo M, Edrington TB, et al. Modeling corneal oxygen with scleral gas permeable lens wear. Optom Vis Sci. 2016;93(11):1339-48. 17. Postnikoff CK, Pucker AD, Laurent J, et al. Identification of leukocytes associated with midday fogging in the post-lens tear film of scleral contact lens wearers. Invest Ophthalmol Vis Sci. 2018;60(1):226-33. 18. Guillon M. Basic contact lens fitting. In: Ruben M, Guillon M, eds. Contact Lens Practice. London: Chapman & Hall Medical; 1994. p 587-622. 19. Carney LG. Luminance of fluorescein solutions. Am J Optom Arch Am Acad Optom. 1972;49(3):200-4. 20. Vincent SJ, Alonso-Caneiro D, Kricancic H, et al. Scleral contact lens thickness profiles: the relationship between average and centre lens thickness. Cont Lens Anterior Eye. 2019;42(1):55-62. 21. Vincent SJ, Alonso-Caneiro D, Collins MJ. Optical coherence tomography and scleral contact lenses: clinical and research applications. Clin Exp Optom. 2019;102(3):224-41. 22. Vajzovic LM, Karp CL, Haft P, et al. Ultra-high-resolution anterior segment optical coherence tomography in the evaluation of anterior corneal dystrophies and degenerations. Ophthalmology. 2011;118(7):1291-6. 23. Jiao H, Hill LJ, Downie LE, et al. Anterior segment optical coherence tomography: its application in clinical practice and experimental models of disease. Clin Exp Optom. 2019;102(3):208-17. 24. Walker MK, Bergmanson JP, Miller WL, et al. Complications and fitting challenges associated with scleral contact lenses: a review. Cont Lens Anterior Eye. 2016;39(2):88-96. 25. Efron N, Holden BA. A review of some common contact lens complications. I. The corneal epithelium and stroma. Optician. 1986;192(5057):21-6. 26. Randleman JB, Woodward M, Lynn MJ, et al. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37-50. 27. Temstet C, Sandali O, Bouheraoua N, et al. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract Refract Surg. 2015;41(4):812-20. 28. Klyce SD. Chasing the suspect: keratoconus. Br J Ophthalmol. 2009;93(7):845-7. 29. Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25(7):604-10. 30. Zeiss. Zeiss receives FDA clearance for epithelial thickness mapping for CIRRUS HD-OCT, enabling more detailed assessment of refractive surgery patients. January 29, 2020. 31. Optovue. Optovue first in US to receive FDA clearance for corneal epithelial thickness mapping. January 29, 2020. 32. Li Y, Chamberlain W, Tan O, et al. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J Cataract Refract Surg. 2016;42(2):284-95. 33. Li Y, Tan O, Brass R, et al. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119(12):2425-33. 34. Kanellopoulos AJ, Aslanides IM, Asimellis G. Correlation between epithelial thickness in normal corneas, untreated ectatic corneas, and ectatic corneas previously treated with CXL; is overall epithelial thickness a very early ectasia prognostic factor? Clin Ophthalmol. 2012;6:789-800. 35. Rocha KM, Perez-Straziota CE, Stulting RD, et al. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg. 2013;29(3):173-9. 36. Fuentes E, Sandali O, El Sanharawi M, et al. Anatomic predictive factors of acute corneal hydrops in keratoconus: an optical coherence tomography study. Ophthalmology, 2015;122(8): 1653-9. 37. Cope JR, Collier SA, Srinivasan K, et al. Contact lens-related corneal infections—United States, 2005-2015. Morb Mortal Wkly Rep. 2016;65(32):817-20. 38. Konstantopoulos A, Kuo J, Anderson D, et al. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am J Ophthalmol. 2008;146(4):534-42. 39. Agarwal A, Ashokkumar D, Jacob S, et al. High-speed optical coherence tomography for imaging anterior chamber inflammatory reaction in uveitis: clinical correlation and grading. Am J Ophthalmol. 2009;147(3):413-6. 40. Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clin Optom (Auckl). 2017;9:41-8. 41. Riley C, Young G, Chalmers R. Prevalence of ocular surface symptoms, signs, and uncomfortable hours of wear in contact lens wearers: the effect of refitting with daily-wear silicone hydrogel lenses (senofilcon a) Eye Contact Lens. 2006;32(6):281-6. 42. Kaštelan S, Lukenda A, Salopek-Rabatic J, et al. Dry eye symptoms and signs in long-term contact lens wearers. Coll Antropol. 2013;37 Suppl 1:199-203. 43. Werkmeister RM, Alex A, Kaya S, et al. Measurement of tear film thickness using ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(8):5578-83. 44. Czajkowski G, Kaluzny BJ, Laudencka A, et al. Tear meniscus measurement by spectral optical coherence tomography. Optom Vis Sci. 2012;89(3):336-42. 45. Lim SH. Clinical applications of anterior segment optical coherence tomography. J Ophthalmol. 2015;2015:605-729. 46. Ibrahim OM, Dogru M, Takano Y et al. Application of Visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010;117(10):1923-9. 47. Zhou S, Li Y, Lu AT, et al. Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: a pilot study. Ophthalmic Surg Lasers Imaging. 2009;40(5):442-7. 48. Bujak MC, Yiu S, Zhang X, et al. Serial measurement of tear meniscus by FD-OCT after instillation of artificial tears in patients with dry eyes. Ophthalmic Surg Lasers Imaging. 2011;42(4):308-13. 49. Ibrahim OM, Dogru M, Kojima T, et al. OCT assessment of tear meniscus after punctal occlusion in dry eye disease. Optom Vis Sci. 2012;89(5):E770-6. 50. Cui X, Hong J, Wang F, et al. Assessment of corneal epithelial thickness in dry eye patients. Optom Vis Sci. 2014;91(12):1446-54. 51. Napoli PE, Coronella F, Satta GM, et al. A simple novel technique of infrared meibography by means of spectral-domain optical coherence tomography: a cross-sectional clinical study. PLoS One. 2016;11(10):e0165558. |

