 |
| Optic neuropathy is a potential side effect of interferon use. |
Its modes of transmission include sexual contact, needle sharing, blood transfusion and transplacental passage from mother to neonate.3 Acute HBV infection is associated with general malaise, fever, loss of appetite, vomiting and jaundice.3 Fulminant presentation of infection involves liver failure accompanied by tissue necrosis.3 The virus is able to inject itself into the host cell to replicate with a resulting immunological response, causing hepatocellular injury.4-6 Common ocular sequellae include ischemic retinopathy, pupil sparing third nerve palsy, optic neuritis and uveitis.7-11
Pertinent Anatomy
Hepatitis B is a DNA virus.12 It is comprised of hepatitis B core antigen (HBcAg), hepatitis B e-antigen (HBeAg), DNA polymerase and double-stranded DNA.13 The entire content of HBV is encapsulated by an envelope that houses the hepatitis B surface antigen (HBsAg).11
Viruses are obligate intracellular parasites—they rely solely on host machinery for replication. Viral tropism refers to the ability of viruses to infect specific cells. When the virus latches onto the host cell, it integrates into the cytoplasm in one of three ways: direct translocation, endocytosis or viral fusion to cell membrane.14 Once inside the cell, viruses start replicating with the help of host enzymes. The newly synthesized viral genome and capsid proteins are assembled into virions, either in the nucleus or cytoplasm, which are then released by budding through the plasma membrane.14
Epidemiology
Of the two billion living people who contracted the virus, approximately 400 million still actively suffer from chronic HBV.15-17 The virus is hyperendemic in regions such as Sub-Saharan Africa, Southeast Asia, China and the West Pacific.13,18
Pathogenesis
HBV mainly infects hepatocytes. The virus enters the body following an exposure to the virus particle through either infected blood or bodily fluids. The hepatocyte uncoats itself and transforms into a covalently closed circular form of DNA (cccDNA).13
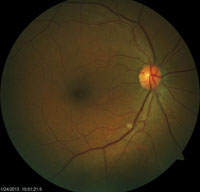 |
| Examination of this patient revealed an isolated cotton wool spot. Upon subsequent lab testing, it was confirmed that this patient had hepatits B. |
Lastly, CD8+ cells release cytokines, such as interferon gamma, which summon macrophages. The massive macrophage response then leads to liver damage.4,5 HBV is not directly cytopathic; instead, it induces an immune reaction that leads to liver injury.13
Initially, there is a marked increase in HBsAg and HBcAg upon infection.14 Once the body detects foreign antigens, antibodies such as immunoglobulin (IgG and IgM) are produced to fight the infection. HBeAg can be detected when viral activity peaks. The infection stage concludes when HBeAg antibodies are formed.18
Chronic infection is defined by the presence of HBsAg in the serum for a period lasting longer than six months.18 During the chronic phase, IgG is still produced to fight the infection—even while the immunity is taxed. In this stage, remaining hepatocyte cells continually divide, and increase the risk for hepatic carcinoma.6
Finally, circulating antigen-antibody complexes have the potential to cause further complications by producing vasculitis, glomerulonephritis and cryogloblinemia.10,19 Ocular manifestations of HBV are the result of these circulating immune complexes, and include various degrees of retinal ischemia, which provoke vasculitis (artery occlusion, vein occlusion, cotton wool spots), pupil sparing third nerve palsy, optic neuritis and uveitis.7-11
Systemic Symptoms
Systemic manifestations of HBV infection during the acute phase of the disease include nausea, vomiting, diarrhea, abdominal discomfort, decreased appetite, fatigue, fever, myalgia, dark urine, and yellowing of the skin and eyes (jaundice).20,21
Ocular Manifestations of Infection
• Retinal vasculitis. The term vasculitis refers to the pathologic inflammation of blood vessels resulting from a buildup of cellular debris secondary to foreign invasion by either virus or bacteria. Retinal vasculitis can be induced by the accumulation of intraretinal inflammatory debris secondary to circulating HBV infection, and the upregulation of cellular elements to eliminate it from the body.22
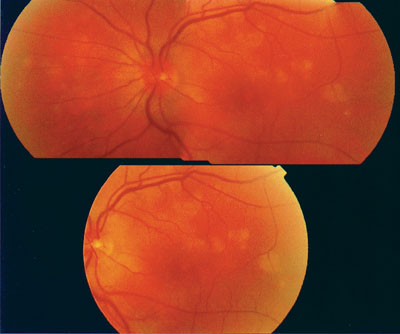 |
| This fundus photo demonstrates the active phase of MEWDS, which is typically seen within 24 hours following hepatitis vaccination. |
Another study determined that hepatitis C virus (HCV) similarly had the potential to induce vasculitis and ischemic retinopathy.7 A separate research group completed a clinical trial of 85 patients and observed that 51% of individuals with HCV exhibited bilateral ischemic retinopathy.24 The clinical features of ischemic retinopathy included cotton wool spots (CWS) and retinal hemorrhages.23 Through their research, they confirmed that the ocular sign of CWS denotes significant ischemia in the affected region. It also provides a palpable sign, indicating ischemic processes are proceeding in the body at large.7,23,24
Herpetic retinal vasculitis (acute retinal necrosis [ARN] and progressive outer retinal necrosis [PORN]) and collagen vascular diseases (CVD) share similar presentations.24 Both of these conditions cause hypoxia secondary to accumulation of inflammatory debris, which impedes retinal perfusion.23,24 Examples of CVD include systemic lupus erthromatosis, Beçhet’s disease, rheumatoid arthritis, sarcoidosis, Sjögren’s syndrome and Reiter’s syndrome. Other conditions, such as anemia, giant cell arteritis, antiplatelet antibody syndrome, diabetes and hypertension, also are capable of such vasculopathy.23
• Pupil-sparing third nerve palsy. A literature review uncovered one case in which a 36-year-old man presented with an acute, pupil-sparing third nerve palsy.8 Evidence of jaundice and darker urine prompted the clinical investigators to perform enzyme linked immuno-assay (ELISA) testing, which uncovered the presence of HBsAg and IgM. The publication concluded that the deposition of circulating immune complexes produced ischemic infarction of the third nerve.8
• Optic neuritis and uveitis. Both optic neuritis and general uveitis have also been observed as consequences of HBV infection.8,10,11,26 A study conducted in Switzerland indicated that 13% of uveitis cases may, in part, be attributed to HBV infection––with evidence of HBsAg circulating within affected individuals’ bloodstreams.11,26 It is theorized that persistent HBsAg and HBcAg in the system leads to constant production of antibodies, resulting in antigen-antibody complex formation, activating the inflammatory system, and eventually causing tissue destruction.27 Moorfields Eye Hospital in London has supported the potential for HBV-based uveitis.11
Work-Up
ELISA testing is essential for detecting circulating hepatitis viral proteins—specifically HBsAg, HBeAg and HBeAg antibodies.18 It is worth noting that the presence of HBsAg in the blood serum for more than six months is an indication of chronicity.18
A complete blood count with platelets is also useful for detecting other concurrent systemic diseases. Additionally, prothrombin time (PT) is helpful to assess the coagulability of the blood, and may indicate liver damage.28
Liver function tests (LFT) are useful to determine the extent of liver damage.29 The battery of liver function tests include alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), albumin, total proteins, bilirubin, gamma-glutamyltransferase (GGT) and L-lactate dehydrogenase (LD).29 A number of results may be indicative of liver damage, including:28
- Elevated ALT, AST, ALP, bilirubin, GGT and LD.
- Reduced albumin and total protein levels.
- Prolonged PT, indicating clotting problems.
In the case of optic neuritis and uveitis secondary to immune complex deposition, be sure to test for:30
- Titrated levels of IgG, IgA, IgM, C3 and C4 on commercial plates.
- Complement haemolytic activity (CH50).
- C1q binding (C1qBA) and conglutinin binding competition (KgB-CA, CIC) assay.30 Raised levels of C1qBA and CIC point to an immune complex etiology, which, as mentioned earlier, also is associated with systemic diseases such as vasculitis, arthritis and glomerulonephritis.30
If vasculitis is observed, additional tests, such as erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), urine tests, imaging (X-ray, CT and MRI) of larger vessels, angiogram and biopsy, should be ordered.31
Treatment for HBV
• Interferon therapy is administered subcutaneously. It modifies the HBV specific response and reduces viral replication.13,14 Interferon is a cytokine released by CD8+ T lymphocytes, which recruits macrophages to the site of the insult.15 Recent clinical trials have indicated that the pegulated form of interferon is the most efficacious.13 The addition of polyetheylene glycol (PEG) to interferon increases the preparation’s half-life and duration of activity.14 Pegulated interferon is administered weekly for up to 48 weeks.
Side effects include headache, myalgia, alopecia and fatigue.14 The most common ocular complication associated with interferon therapy is retinal ischemia, which is characterized by CWS, microvascular abnormalities and hemorrhages.32-34
Patients placed on the medication should be monitored for fundus changes, visual field abnormalities and retinal nerve fiber layer (RNFL) thickness alterations every three to four months following dosing initiation.33 Increased RNFL thickness warrants close observation, and any patient who manifests CWS should cease therapy immediaely.34
Other, less common conditions associated with interferon use include subconjunctival hemorrhage, retinal detachment, optic neuropathy and elevated intraocular pressure.29,30,35 Ocular signs typically present from two weeks to six months following therapy initation.29
• Nucleotide analogs reduce HBV replication by mimicking and inserting themselves as a base upon the viral DNA, effectively halting HBV replication.13,14 In comparison to interferon, these agents are administered orally. Current analogs substantially reduce the amount of HBV DNA, but have not demonstrated the ability to completely eradicate the virus.
This catagorizes the medication as a drug for maintaining viral suppression.13
Lamivudine is an effective drug for patients with cirrhosis and recurrent hepatitis after liver transplant.17 The main disadvantage of the medication is viral resistance. Entecavir can be used as an alternative when resistance to lamivudine is detected.17
• Vaccination has dramatically reduced the prevalence of HBV around the globe.13 The vaccination is an effective agent that is safe for administration at birth.2 The modality can be used prophylactically to prevent perinatal transmission and has been shown to be 89% to 98% effective.2 Data has shown a large decline in the rate of chronic disease occurrence in the children of Taiwan over a 10-year period (9.8% to 1.3%) following the inception of a successful HBV vaccination program.13
The HBV vaccine is comprised of purified HBsAg and is produced via recombinant DNA technology, and typically is administered in three doses.19 It is is the most widely used vaccine in the world, and is now being considered as the standard of care in the United States.2 Furthermore, the World Health Organization (WHO) is also pushing for vaccination in hyperendemic areas.13
Hepatitis vaccination is not free from potential side effects. There have been a total of 32 uveitis cases resulting from use of the hepatitis B vaccination.2 Episodes of acute uveitis relating to immune complex sequellae also have been documented.36,37
Many reports of uveitis relating to HBV have been published between 1982 and 2009 from databases like the National Registry of Drug-Induced Ocular Side Effects, The World Health Organization and the FDA.2 Uveitis is often seen after the first vaccination, and typically the manifestation ensues at day three or later.2 Recurrence of uveitis after the second and third vaccinations are rare.2 The ocular treatment for cases of uveitis from this source are standard (cycloplegia and topical steroidal anti-inflammatory drops), with patients typically responding well to the intervention.
Posterior uveitis also has been reported––specifically multiple evanescent white dot syndrome (MEWDS).34 The condition typically is seen within a window of 24 hours following vaccination.36 This clinical presentation may be associated with increased levels of IgG and IgM.34 The ocular treatments for cases of MEWDS from this source are also standard (cycloplegia and topical anti-inflammatory drops), with patients responding well to the intervention.34
Other ophthalmological symptoms, such as disc edema, central vein occlusion and optic neuritis, may be seen after vaccination.34
HBV infection is the leading cause of liver cancer around the globe. It is hyperendemic, especially in densely populated areas such as Africa, Southeast Asia, China and the West Pacific. The formation of immune complexes due to viral insult can be catastrophic to ocular health, and can result in debilitating diseases, such as vasculitis induced retinal ischemia, optic neuritis, uveitis and pupil sparing third nerve palsy.
Eye care professionals have the potential to play a key role in the diagnosis and management of HBV as well as in its monitoring after treatment by detecting ocular signs such as isolated cotton wool spots and/or retinal hemorrhages. In cases exhibiting these signs where the etiology is unexplained, prompt investigation of the underlying cause through laboratory testing is required. When patients are managed preemptively by vaccination, or currently by medications, close monitoring is warranted, due to risk of ocular complications. The team approach between the eye care professional and the general practitioner is crucial when managing HBV.
Dr. Tuyen is on staff at Eye Physicians and Surgeons and is currently a clinical consultant at The Eye Institute. Dr. Gurwood is a professor at Salus University in Elkins Park, Pa.
1. Hadziyannis SJ, Papatheodoridis GV. Hepatitis b e antigen-negative chronic hepatitis b: Natural History and Treatment. Seminars in Liver Disease. 2006 May;26(2):130-41.
2. Shepard CW, Simard EP, Finelli L, Fiore AE, et al. Hepatitis B virus infection: epidemiology and vaccination. Epidemiologic Reviews. 2006 Jun;28(1):112-25.
3. Chandrasoma, Para, Taylor CR. Concise Pathology. Norwalk, CT: Appleton & Lange; 1995. Chapter 42; 631-6.
4. Chisari FV, Ferrari C. Hepatitis b virus immunopathogenesis. Annual Review of Immunology. 1995;13(1):29-60.
5. Chisari FV, Ferrari C. Hepatitis b virus immunopathology. Springer Seminars in Immunopathology. 1995;17(2-3):261-81.
6. Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis b virus infection. Pathologie Biologie. 2010 Aug;58(4):258-66.
7. Zegan ME, Anninger W, Chapman C, Gordon SR. Ocular manifestations of hepatitis c virus infection. Current Opinion in Ophthalmology. 2002 Dec;13(6):423-7.
8. Sood A, Midha V, Sood N, Gupta D. Hepatitis b and pupil‐sparing oculomotor nerve paresis. Clinical Infectious Diseases. 1999 Nov;29(5):1330-1.
9. Farthing CF, Howard RS, Thin RN. Papillitis and hepatitis b. Bmj. 1986 Jun;292(6537):1712.
10. Bloom JN, Rabinowicz M, Schulman ST. Uveitis complicating autoimmune chronic active uveitis. Am J Dis Child. 1983 Dec; 137(12):1175-6.
11. Murra PI, Prasad J, Rahi AH. Status of hepatitis b virus in the aetiology of uveitis in great britain. British Journal of Ophthalmology. 1983 Oct;67(10):685-7.
12. Sonneveld MJ, Zoutendijk R, Janssen HLA. Hepatitis b surface antigen monitoring and management of chronic hepatitis b. Journal of Viral Hepatitis. 2011 Jul;18(7):449-57.
13. Ocama P, Opio C, Lee W. Hepatitis b virus infection: current status. The American Journal of Medicine. 2005;118(12):1413.e15-413.e22.
14. Cotran, Ramzi S, Kumar V, Collins T, Robbins SL. Robbins Pathologic Basis of Disease. Philadelphia: Saunders; 1995. Chapter 19.
15. Buster EHCJ, Janssen HLA. Antiviral treatment for chronic hepatitis b virus infection-immune modulation or viral suppression. The Netherlands Journal of Medicine. 2006 Jun;64(6):175-85
16. Kane M. Global programme for control of hepatitis b infection. Vaccine. 1995;13 Suppl 1:S47-9.
17. Lee WM. Hepatitis b virus infection. N Engl J Med. 1997 Dec;337(24):1733-745.
18. Ching LL, Ratzlu V, Yuen MF, Poynard T. Viral hepatitis b. Lancet. 2003 Dec;362(9401):2089-94.
19. Rubin, Emanuel, Gorstein F. Rubin’s Pathology: Clinicopathologic Foundations of Medicine. Philadelphia: Lippincott Williams & Wilkins; 2004. Viral hepatitis; 762-72.
20. Fraunfelder FW, Eric BS, Fraunfelder FT. Hepatitis b vaccine and uveitis: an emerging hypothesis suggested by review of 32 case reports. Cutaneous and Ocular Toxicology. 2010 Mar;29(1):26-9.
21. Chambers RB, Downie A, Foote B, Davidorf FH. Interferon alfa-associated retinopathy. JAOA. 1997 Jan;97(1):43-5.
22. Gower RG, Sausker WF, Kohler PF, Thorne GE, et al. Small vessel vasculitis caused by hepatitis b virus immune complexes. Journal of Allergy and Clinical Immunology. 1978 Oct;62(4):222-8.
23. Dieter S. The mystery of cotton-wool spots a review of recent and historical descriptions. Eur J Med Res. 2008 Jun;13(6):231-66.
24. Abe T, Nakajima A, Satoh N, Koizumi T, et al. Clinical characteristics of hepatitis c virus-associated retinopathy. Jpn J Opthalmol. 1995;39(4):411-9.
25. Wensing B, De-Groot Mijnes JD, Rothova A. Necrotizing and nonnecrotizing variants of herpetic uveitis with posterior segment involvement. Arch Opthalm. 2011 Apr;129(4):403-8.
26. Grob PJ, Martenet AC, Witmer R. Nonspecific immune parameters and hepatitis b antigens in patients with uveitis. Mod Probl Ophthalmol. 1976;16:254-8.
27. London WT. Hepatitis b virus and antigen-antibody complex disease. N Engl J Med. 1977;296:1528-9.
28. Mayo Clinic. Liver function tests. Available at: http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/definition/prc-20012602. Accessed May 12, 2013.
29. Nussenblatt, Robert B, Whitcup SM. Uveitis: Fundamentals and Clinical Practice. St. Louis: Mosby; 2004. Diagnosis:76-87
30. Galli M, Morelli R, Casellato A, Perna MC. Retrobulbar optic neuritis in a patient with acute type b hepatitis. Journal of the Neurological Sciences. 1986;72(2-3):195-200.
31. Mayo Clinic. Vasculitis. Available at: http://www.mayoclinic.org/diseases-conditions/vasculitis/basics/definition/con-20026049. Accessed May 12, 2013.
32. Manesis EK, Moschos M, Brouzas, D, Kotsiras J, et al. Neurovisual impairment: a frequent complication of alpha-interferon treatment in chronic viral hepatitis. Hepatology. 1998 May; 27(5):1421-7.
33. Guyer DR, Tiedeman J, Yannuzi LA, Slakter JS, et al. Interferon-associated retinopathy. Arch Opthalm. 1993 Mar;111(3):350-56.
34. Koktekir BE, Sumer S, Bakbak B, Gedik S, et al. Ocular effects of pegylated interferon alpha in patients with chronic hepatitis b. Cutaneous and Ocular Toxicology. 2013 Oct;32(4):275-8.
35. Hayasaka S, Masamitsu F, Yamamoto Y, Noda S, et al. Retinopathy and subconjunctival hemorrhage in patients with chronic viral hepatitis receiving interferon alpha. British Journal of Ophthalmology. 1995;79(2):150-52
36. Baglivo E, Safran AB, Borruat FX. Multiple evanescent white dot syndrome after hepatitis b vaccine. Brief Reports. 1996 Sep;122(3):431-32.
37. Muferet E, Guven S, Akyuz U, Bilgiç O, et al. Optic neuritis following hepatitis b vaccination in a 9-year-old girl. Journal of the Chinese Medical Association. 2009 Nov; 72(11):594-97.

