 |
A 53-year-old Hispanic male presented for a second opinion on a previous diagnosis. He reported vision loss in his right eye that had persisted for more than 20 years. His medical history was unremarkable.
The patient was told that “he had pressure in his eye,” but admitted that he didn’t really understand the diagnosis. He suggested that the vision in his right eye had been fairly stable over the past several years since he moved to the United States.
On examination, his best-corrected visual acuity measured 20/400 OD and 20/20 OS. Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting OU. Pupils were equally round and reactive, with trace evidence of afferent defect in his right eye. The anterior segment was unremarkable. His intraocular pressure measured 15mm Hg OU.
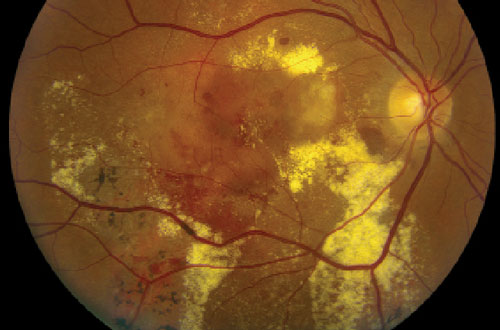
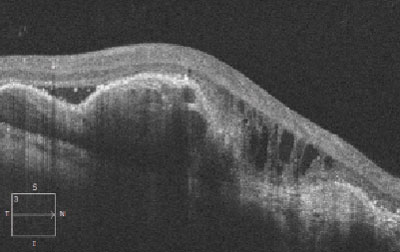
Dilated fundus exam revealed healthy optic nerves, with good rim coloration and perfusion. We noted significant retinal changes in his right eye (figure 1). We also noted changes in the left eye (figure 2). Additionally, we ordered a spectral-domain optical coherence tomography (SD-OCT) scan (figure 3).
|
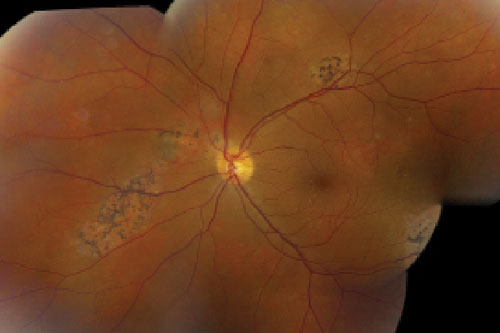 |
| Figs. 1 & 2. Fundus image of his right eye (top), and widefield view of his left eye (bottom). How do you explain the pigmentary anomalies? |
Take the Retina Quiz
1. How would you characterize the SD-OCT findings documented in his right eye?
a. Retinal pigment epithelium (RPE) tear.
b. Exudative retinal detachment.
c. Combination pigment epithelial detachment (PED) and serous detachment.
d. Macular schisis.
2. How do you account for the areas of RPE hypertrophy OU?
a. Retinal degeneration from early-stage retinitis pigentosa (RP).
b. Unreported trauma.
c. Previous episodes of retinal edema and fluid.
d. Previous toxoplasmosis.
3. What is the correct diagnosis for this patient?
a. Wet macular degeneration.
b. Coats’ disease.
c. Polypoidal choroidal vasculopathy (PCV).
d. Branch retinal vein occlusion with subretinal neovascularization.
4. How should this patient likely be treated?
a. Laser photocoagulation.
b. Photodynamic therapy (PDT).
c. Anti-VEGF injection.
d. Combination PDT and anti-VEGF therapy.
For answers, scroll to the bottom.
Discussion
There is massive exudation and subretinal hemorrhage throughout the posterior pole of our patient’s right eye. The SD-OCT shows an irregular pigment epithelial detachment with an overlying serous retinal detachment, as well as cystoid macular edema OD.
It would be easy to assume that these findings, in conjunction with the subretinal hemorrhage, are due to a large, occult choroidal neovascular membrane. But, that is not the likely cause. Instead, he has PCV.
Lawrence A. Yanuzzi, MD, first described polypoidal choroidal vasculopathy in 1982.1 The hallmark feature of PCV is a network of vessels located in the inner choroid that develop peculiar aneurismal dilations. The disease is characterized by multiple, recurrent, serosanguineous detachments of the RPE and neurosensory retina secondary to leakage and bleeding from these aneurismal choroidal vascular lesions.1-3 In fact, one of the earliest names for the condition was “posterior uveal bleeding syndrome,” because of the massive amount of hemorrhaging and exudation. Looking at our patient, you can understand why.
In the earlier disease stages, you can sometimes see the aneurismal polyp-like lesions located below the RPE. These appear as reddish-orange, spheroidal lesions. Over time, these vessels can slowly begin to bleed with varying amounts of exudation. Indocyanine green angiography (IGC) may be the definitive test for establishing a diagnosis of PCV, as the polyp-like aneurysmal dilations are clearly highlighted.
| |
|
|
Fig. 3. Spectral-domain optical coherence tomography scan through the macula of our patient’s right eye. What is the underlying etiology? |
Keep in mind that PCV easily can be misdiagnosed for wet macular degeneration. In one study of 167 consecutive patients diagnosed with wet AMD, the researchers determined that 13 patients (7.8%) actually had PCV.4 Additionally, PCV tends to present in more darkly pigmented individuals (i.e., blacks, Hispanics and Asians), compared to AMD, which is more frequently diagnosed in whites.
PCV also tends to occur in younger individuals––50 to 65 years of age, compared to 65+ for AMD.3 Our patient is 53, much younger than the typical AMD patient. Finally, AMD is associated with drusen, which typically are not seen in younger PCV patients.
The changes seen in our patient’s left eye are also quite revealing. We noted localized areas of bony pigment spiculing, which likely is due to previous PCV activity. It appears that the patient developed scarring from resolved episodes of bleeding, exudation and retinal edema. But, because these features did not involve the macula, the patient remained completely unaware of any underlying disease process.
Photodynamic therapy has emerged over thermal laser as the treatment of choice for PCV. However, more recently, researchers have investigated the clinical efficacy of intravitreal anti-VEGF therapy.
The EVEREST study was a multicenter, double-masked trial comparing the therapeutic effect of PDT plus Lucentis (ranibizumab, Genentech/Roche), PDT monotherapy, and Lucentis monotherapy on polypoidal choroidal vasculopathy.5 The six-month results indicated that PDT plus Lucentis and PDT monotherapy were superior to Lucentis treatment alone in achieving complete polyp closure regression (77.8% and 71.4% vs. 28.6%, respectively). Eyes treated with PDT plus Lucentis also achieved better visual acuity. Specifically, patients who underwent combination PDT and Lucentis gained 10.9 ETDRS letters, those who received Lucentis monotherapy gained 9.2 letters and those who underwent PDT monotherapy gained 7.5 letters.5 It is important to note that patients who received PDT (either alone or in combination) exhibited better PCV resolution than those who received Lucentis monotherapy.5
Given these findings, it seems that combination PDT and Lucentis therapy may yield better lesion regression and a longer duration of treatment effect than either form of monotherapy.5 A similar study comparing the clinical efficacy of Lucentis and Avastin (bevacizumab, Genentech/Roche) for PCV produced similar results.6
We referred our patient to a retinal specialist for treatment. Considering his 20-year history of vision loss, it doesn’t seem likely that he will recover much central vision. On the other hand, the retinal findings do not look consistent with a process that has been progressing for two decades. So perhaps, if we can get rid of the fluid and flatten his macula, we may be pleasantly surprised at how well he does. Only time will tell.
1. Yannuzzi LA. Idiopathic polypoidal coroidal vasculopathy. Presented at the Macula Society Meeting. February 5, 1982; Miami, Fla.
2. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1-8.
3. Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997 Apr;115(4):478-85.
4. Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy andn neo vascularized age-related macular degeneration. Arch Ophthalmol. 1999 Nov;117(11):1503-10.
5. Koh AH, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013 Apr;33(4):686-716.
6. Cho HJ, Kim JW, Lee DW, et al. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond). 2012 Mar;26(3):426-33.
Retina Quiz Answers
1) c; 2) c; 3) c; 4) d.

