Thyroid-associated ophthalmopathy (TAO), also known as thyroid eye disease or Graves’ ophthalmopathy, is the most common autoimmune inflammatory disorder of the orbit and periorbital tissue, with approximately three million Americans affected.1,2 This prevalence is similar to that of glaucoma in the United States. Historically, TAO was limited to patients with Graves’ disease and the clinical triad of orbital signs, hyperthyroidism and pretibial myxedema.3 Now, research shows only 80% of patients with TAO have Graves’ (hyperthyroidism); the other 20% consists of patients who are hypothyroid (10%) and euthyroid (5% to 10%).4 Because TAO can precede, coincide with or succeed the diagnosis of thyroid dysfunction, optometrists need to be capable of making an early diagnosis, as TAO can be vision-threatening, impact a patient’s appearance and result in loss of quality of life.5,6
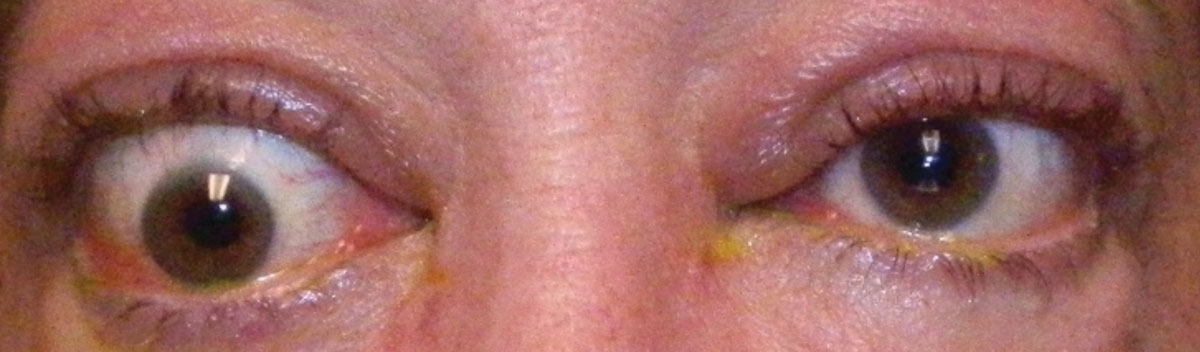 |
| Fig. 1. Hypotropia of the right eye in a patient with TAO. Click image to enlarge. |
Where Exposure Starts
Three broad categories summarize the pathogenesis for TAO: (1) inflammation of the periorbital soft tissue; (2) activation of a subpopulation of orbital fibroblasts that are capable of undergoing adipocyte differentiation, leading to hyperplasia of adipose tissue; and (3) overproduction of glycosaminoglycans by orbital fibroblasts.7 Orbital fibroblasts produce collagen and glycosaminoglycans in the extracellular matrix and create a strong polyanionic charge, which causes an extremely high osmotic pressure, leading to extraocular muscle swelling and possible optic nerve compression.8
TAO presents in two distinct phases: the active inflammatory phase and the inactive, or stable, phase. Typically, the stronger and more aggressive the active phase, the higher the likelihood of more severe sequelae.9 The active inflammatory period typically lasts between six and 24 months and is followed by a quiet, stable, chronic fibrotic period.10 During the early active phase, immunomodulators and radiotherapy may limit the progression of TAO. Once the disease is in the inactive state, surgery may help to improve cosmesis, comfort and function.
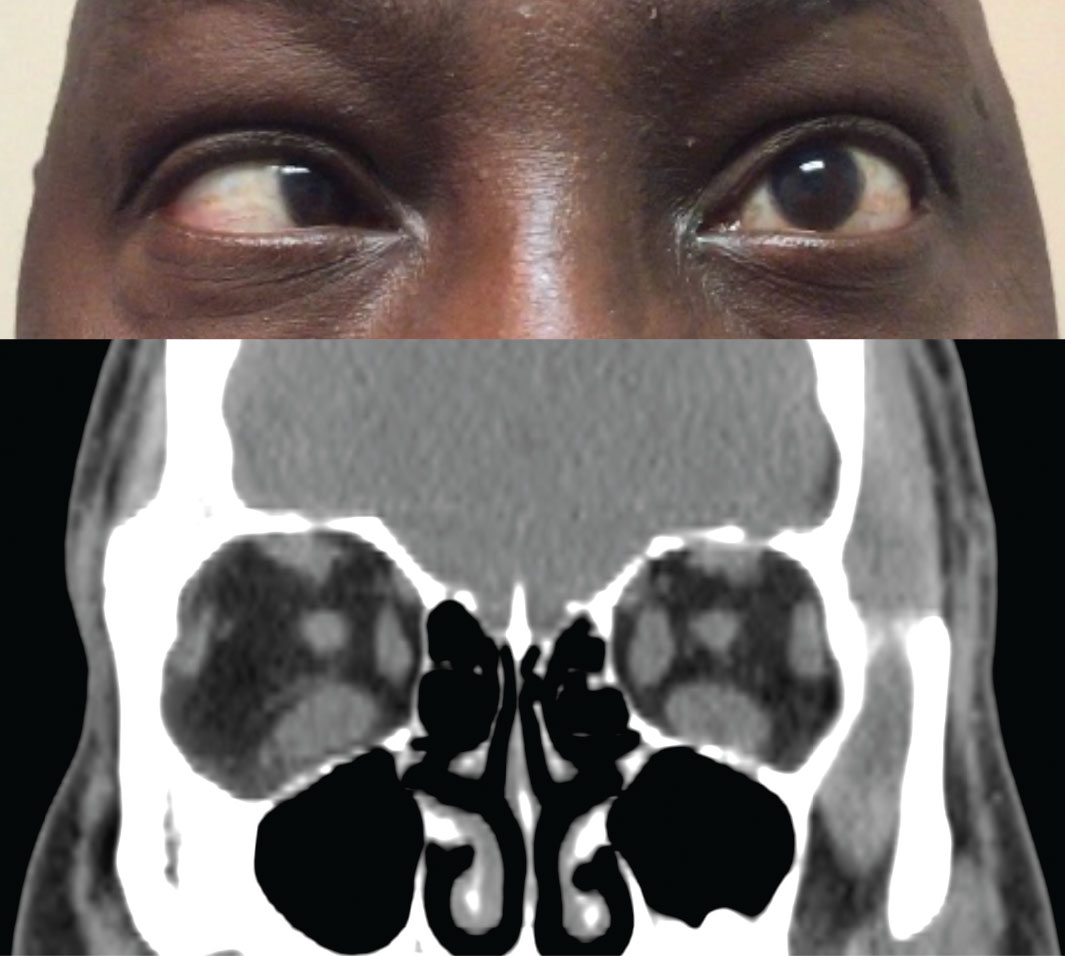 |
| Fig. 2. Above, restricted movement of the left eye in temporal gaze. Below, coronal CT scan of the orbits shows an increased thickness of the left medial rectus and both inferior recti. Click image to enlarge. |
Early Clues
The clinical signs for TAO are vast, with most patients developing eyelid retraction, proptosis and other features of ocular exposure such as epiphora, photophobia, pain, grittiness, diplopia and decreased vision.11 If the inflammation extends to the extraocular muscles, patients can develop conjunctival erythema, chemosis, restrictive extraocular myopathy, hypotropia, double vision and compressive optic neuropathy (Figure 1).
Table 1. TAO Evaluation | |
| Clinical Exam |
|
| Laboratory Tests |
|
| Imaging |
|
You can make the diagnosis of TAO based on presenting ocular signs and symptoms. Changes in appearance and exposure symptoms are the most common early findings in TAO.9 Other symptoms are vague and often attributed to normal aging, especially in middle-aged women with eyelid swelling that is worse in the morning.12
The most common sign in TAO is lid retraction, which occurs in 82% of patients.13 This may be due to increased sympathetic tone, overaction of the levator and superior rectus muscles to compensate for inferior rectus restriction, or inflammation and scarring of the levator complex (Figure 2).
The second most common sign is proptosis (62%), which is caused by expansion of the orbital fat, muscles or both (Figure 3). Proptosis can be measured with an exophthalmometer. Values greater than 18mm to 20mm for Caucasians, 16mm to 18mm for Asians and 20mm to 22mm for African-Americans suggest proptosis. Asymmetry of 2mm or greater also suggests proptosis.14,15 If a clinician does not have an exophthalmometer, orbital CT scans can evaluate the amount of proptosis as well.
Proptosis and eyelid retraction can lead to increased corneal exposure and ocular surface disease—a common reason why patients make an eye appointment. If the disease state is aggressive, the proptosis and lid retraction can lead to lagophthalmos, exposure keratopathy, microbial keratitis and perforation. Patients at risk for perforation typically have significant lagophthalmos and an absence of Bell’s phenomenon due to fibrosis of the inferior rectus muscle.
Restrictive extraocular myopathy (42%) and optic nerve dysfunction (6%) are also likely signs.13 Dysthyroid optic neuropathy, sight-threatening but potentially reversible, should be suspected with desaturation of colors, afferent pupillary defects or decreased vision.9 Most cases of dysthyroid optic neuropathy present with muscle enlargement but not necessarily severe proptosis.9 Some believe patients with tight eyelids have limited anterior movement of the globe, putting them at greater risk for compression of the optic nerve.
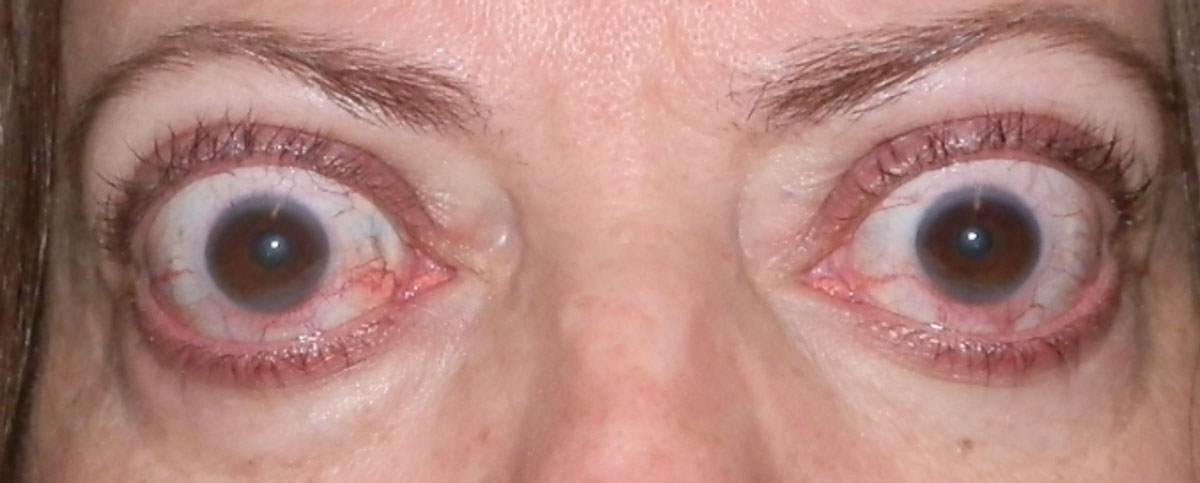 |
| Fig. 3. Upper eyelid retraction and proptosis in a patient with orbital congestion (conjunctival injection) in the active phase. Click image to enlarge. |
Other signs include temporal flare, conjunctival injection and chemosis, Von Graefe’s sign (delayed eyelid lag during downgaze) and Dalrymple’s sign (widening of the palpebral fissures). Clinical signs are typically bilateral and symmetric but can be asymmetric or unilateral. In these cases, asking about symptoms of thyroid dysfunction—such as hair loss, heat or cold intolerance, weight changes, skin changes, memory problems and changes in their mood—can help make the diagnosis (Table 1).
Asymmetric Presentations
When you suspect a unilateral presentation and the patient reports little or no systemic symptoms, consider orbital imaging and thyroid serum testing. If you discover normal or mildly abnormal thyroid functions, consider alternate etiologies (Figure 4).16
The most common differential of TAO is orbital pseudotumor or non-specific orbital inflammation.17 Non-specific orbital inflammation can present with TAO signs such as proptosis, extraocular muscle restriction, red eye and chemosis. Non-specific orbital inflammation is usually acute and painful, but if the clinical picture is unclear, orbital imaging can assist in distinguishing between these different disease processes.16 For example, on CT scan TAO demonstrates the pathognomonic pattern of extraocular muscle enlargement while sparing the muscle tendon. Orbital pseudotumor, however, demonstrates enlargement of both the extraocular muscle and tendon.9
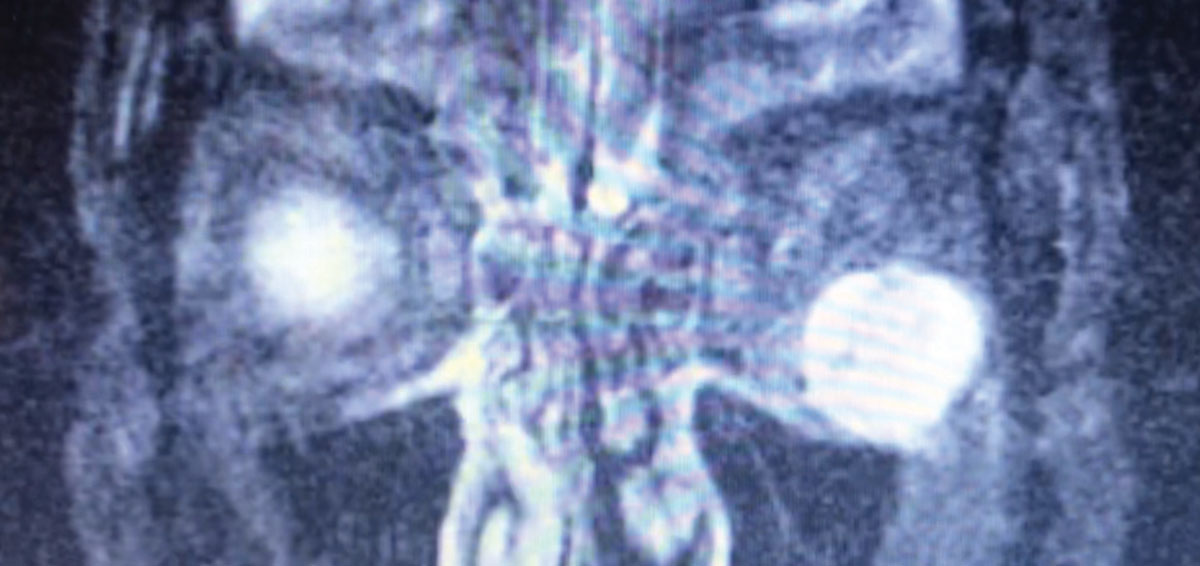 |
| Fig. 4. This patient presented with unilateral proptosis. Exam findings suggested TAO; however, further testing, including this MRI, revealed an inferolateral postseptal orbital mass (hyperintense here) abutting the inferior and lateral rectus muscles. These features were highly suggestive of an orbital cavernous venous malformation (hemangioma). Click image to enlarge. |
Keeping Score
Several clinical scoring systems exist for assessing the severity of TAO, including the mnemonics NO SPECS and VISA. These systems can help guide your evaluation and treatment of patients with TAO, but currently no system prevails as the gold standard.
Most clinicians know the classic NO SPECS classification: No physical signs or symptoms, Only signs, Soft tissue involvement, Proptosis, Extraocular muscle involvement, Corneal involvement and Sight loss (due to optic nerve compression).18 While helpful, it does not assess clinical activity, nor does it provide sufficient information to document the disease between visits as a means for guiding management.9,19
Table 2. Clinical Activity Score | |
| Retrobulbar pain | 0-1 |
| Pain on extraocular muscle movement | 0-1 |
| Eyelid erythema | 0-1 |
| Conjunctival injection | 0-1 |
| Chemosis | 0-1 |
| Inflammation of caruncle | 0-1 |
| Eyelid edema | 0-1 |
| Total | 0-2: inactive TAO 3-7: active TAO |
The VISA classification—Vision, Inflammation, Strabismus and Appearance—was developed to permit grading of both clinical severity and activity based on both subjective and objective inputs (Figure 5).20 This system helps direct appropriate management in a logical sequence by targeting the most relevant aspect of the disease affecting the patient. For example, vision dysfunction from the optic nerve is the first priority. VISA is also beneficial in assessing TAO and grading changes and can act as a guide for therapy.20 The International Thyroid Disease Society has adopted it, and it is used in recent clinical trials.
Another popular method grades TAO from mild to severe.21 The European Group on Graves’ Orbitopathy (EUGOGO) defines mild disease as minimal eyelid swelling, lid retraction or proptosis with little or no extraocular muscle dysfunction. Moderate to severe TAO consists of some form of active disease with or without ocular motility dysfunction with diplopia and inflammatory features interfering with the ability to function. It may also include significant proptosis. Serious disease refers to sight-threatening conditions such as dysthyroid optic neuropathy and corneal ulceration.9
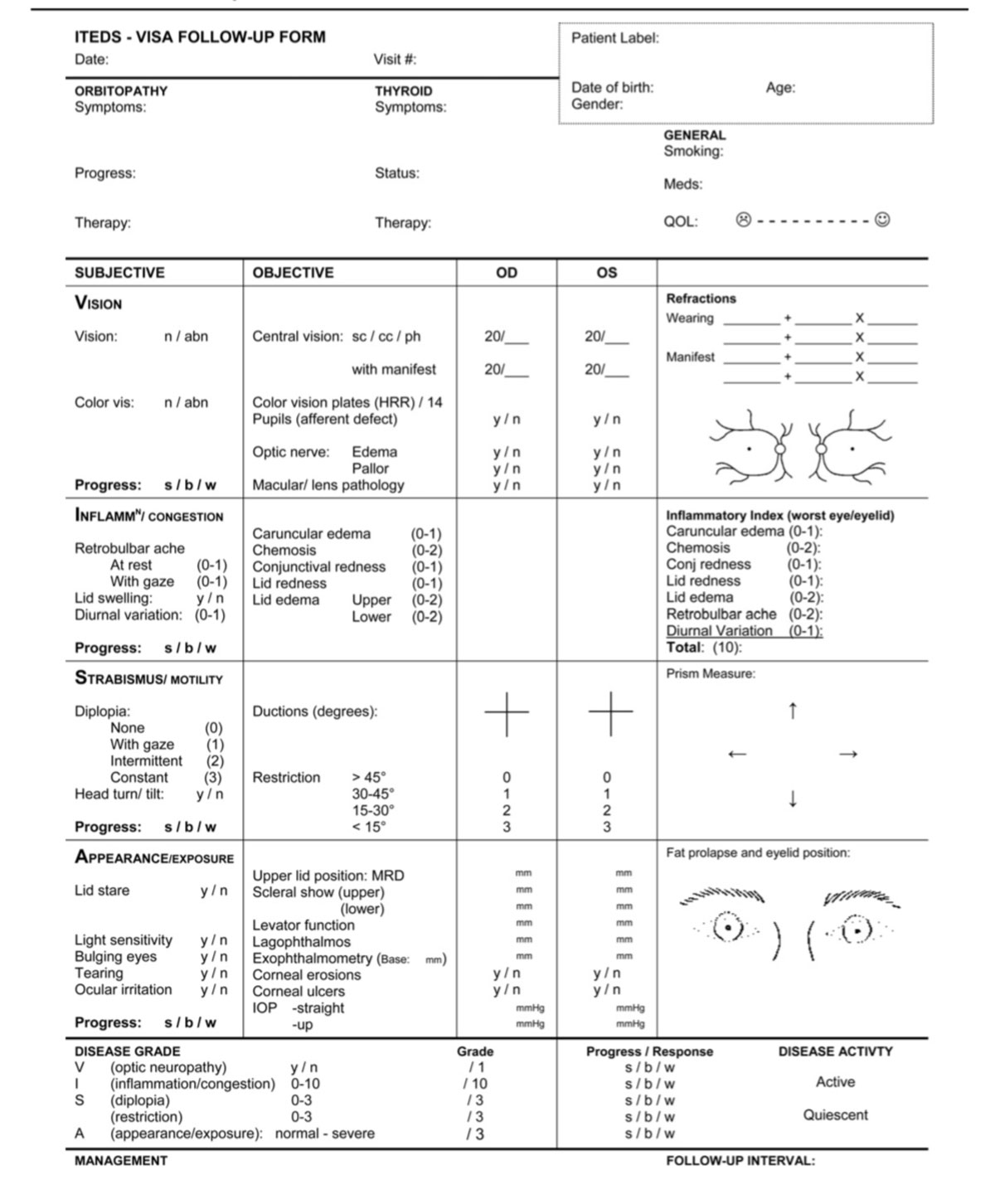 |
| Fig. 5. The VISA classification form allows clinicians to monitor the severity and the activity of TAO. Click image to enlarge. |
Timing is Everything
Evaluating the severity of orbital changes when diagnosing TAO is important, but this provides only a snapshot of the condition. Equally important is a temporal assessment of the course of the disease state and where it lies on Rundle’s curve.
The key to determining if a patient with TAO is in a sight-threatening situation is assessing if the patient is in the active inflammatory phase or the stable, latent chronic fibrotic phase.
Active. Most patients who suffer vision loss from TAO due to corneal exposure or compressive optic neuropathy do so during the active inflammatory phase. You can use the clinical activity score (CAS) to identify active disease (Table 2).18,22 Periorbital erythema and edema, conjunctival injection, chemosis, orbital inflammation and congestion, eyelid retraction, proptosis and diplopia typically characterize this phase. The active phase is typically a self-limiting disease process that lasts one year in nonsmokers and two to three years in smokers.10
Table 3. Management of Thyroid Eye Disease | |
| Disease Stage | Recommendation |
| Mild |
|
| Moderate |
|
| Severe |
|
| Refractory |
|
When using CAS, note that severe disease complications, such as dysthyroid optic neuropathy, are still possible with low CAS scores, and patients with high CAS scores may have long-standing congestive changes that are unresponsive to any immunotherapy, requiring mechanical surgical decompression.
Latent. After the active phase plateaus, the patient enters the quiescent burn-out phase. This latent, chronic fibrotic phase presents with similar clinical findings as the active inflammatory phase (i.e., eyelid retraction, proptosis and diplopia) but does not have many of the inflammatory signs seen during the active phase (i.e., conjunctival injection and chemosis) (Figure 6).23 These patients require frequent follow-up, as reactivation of inflammation can occur in 5% to 10% of patients over their lifetime.24
On the HorizonWithout any known biomarkers for the disease, thyroid dysfunction is currently detected with serum testing. However, a recent study investigating the tears of patients with TAO identified three new possible biomarkers, suggesting tears could be a source to diagnose TAO early and potentially even identify the amount of inflammation present.25 As for treatment options, researchers recently linked a newly discovered signaling pathway that involves immunoglobulin activation of insulin-like growth factor I (IGF-I) receptor (IGF-IR) in patients with Graves’ disease.31-33 This IGF-I signaling pathway can act synergistically with thyrotropin, enhancing its mechanism of action.33 Studies show that an inhibitory antibody targeting IGF-IR can attenuate the actions of IGF-I, thyrotropin, thyroid-stimulating immunoglobins and immunoglobulins associated with patients with Graves’ disease.34,35 These observations led to the development of a new pharmacological compound called teprotumumab.36 This compound is a fully human IGF-IR-inhibitor monoclonal antibody that can halt the signaling pathway of IGF-I and decrease the body’s sensitivity to thyrotropin, thus lessening the signs and symptoms associated with Graves’ disease.36 This led to the first double-masked, randomized, multicenter, placebo-controlled trial for patients with moderate and severe TAO.36 When compared with placebo, teprotumumab demonstrated improvements in proptosis and other signs and symptoms associated with TAO.36 Though the long-term benefits and safety profile are still under investigation, the results are promising. The FDA has granted this agent the fast-track designation, orphan drug designation and breakthrough therapy designation for Graves’ orbitopathy. These could assist in fast-tracking this medication’s review process through the FDA once the manufacturer submits a biologics license application. You should familiarize yourself with this new potential medication, as it will likely be the standard of care in treating patients with TAO in the near future. |
Treatment Plan
Managing TAO includes restoring euthyroidism and smoking cessation.6 Although euthyroidism is a treatment target, achieving well-controlled thyroid hormone levels will not improve TAO; however, poorly controlled thyroid hormone levels can make it worse. Thus, treatment of the thyroid gland must be simultaneous with but independent of treatment for the ophthalmopathy. Treatment includes a stepwise approach based on patient-reported symptoms, clinical examination and ancillary testing (Table 3).
Fortunately, the active inflammatory phase is usually mild and self-limited, often requiring only supportive intervention (e.g., artificial tears, topical anti-inflammatories). For most mild cases of TAO, simple treatment measures such as lubricants for lid retraction, nocturnal ointments for incomplete eye closure, prisms for diplopia and botulinum toxin injections for upper-lid retraction can be useful.
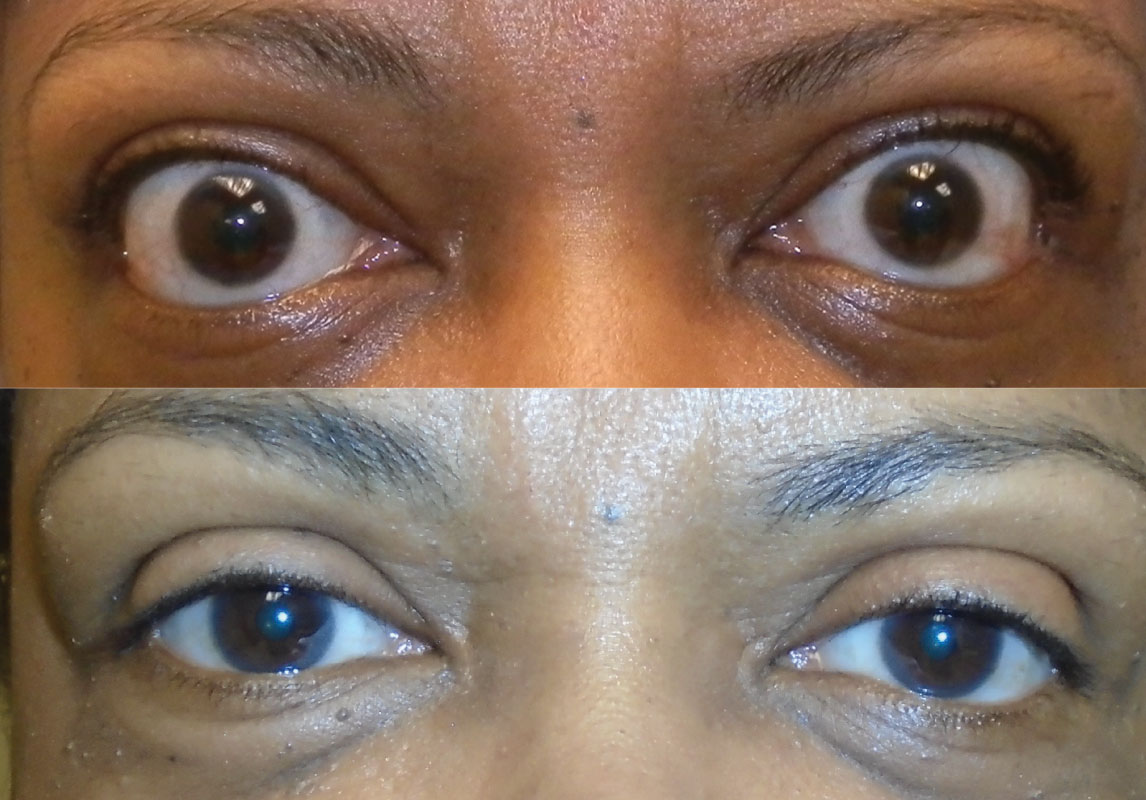 |
| Fig. 6. Before (above) and after (below) orbital decompression of a patient in the stable, inactive phase. Click image to enlarge. |
If the active phase is aggressive, it is essential to administer treatments that can decrease the severity and duration of the active disease process. Glucocorticoids, orbital radiotherapy and decompression/rehabilitative surgery are generally indicated for moderate-to-severe TAO and for sight-threatening optic neuropathy, cornea exposure and spontaneous globe subluxation. Other steroid-sparing immunomodulators, including rituximab, aim to treat the underlying molecular and immunological factors.25
TAO and SmokingPatients with Graves’ disease who smoke have a five-fold higher risk of developing TAO than those who do not smoke.37 When orbital fibroblasts are exposed to cigarette extract, there is a dose-dependent statistically significant increase in glycosaminoglycan production and adipogenesis.38 Evidence also suggests that smoking cessation reduces the severity of TAO and increases the chance of these patients having a favorable response to treatment.39 It is crucial that ODs have a discussion about smoking cessation with all patients with Graves’ and TAO. |
Approximately 5% of TAO patients require some surgical procedure such as elective orbital decompression, eye muscle surgery or eyelid surgery.26 Surgical intervention is best done once the active phase has resolved and the patient has been in the stable chronic fibrotic period for at least six months.10 Complications associated with the use of blepharoplasty in active TAO can result in massive inflammation, orbit muscle involvement and overall unpredictable procedure results.27 Thus, clinicians should rule out early and active TAO before recommending patients for lid surgery.27
It is important to note that medical therapy options for patients with moderate-to-severe TAO are lacking, with no current FDA-approved therapy. High-dose glucocorticoids, orbital radiotherapy and immunomodulators can reduce the amount of ocular and orbital inflammation associated with this disease, but their effect on proptosis is minimal, and their systemic side-effect profile can be high.28-30 Many patients with TAO, despite high-dose glucocorticoid treatment and radiotherapy, do not improve and go on to develop compressive optic neuropathy, corneal exposure or orbital congestion.31,32
Clinicians are still on the hunt for alternative treatment modalities that will overcome the current treatment limitations.
Dr. Saenz is the clinic and residency director at Parkhurst NuVision in San Antonio, Texas and an adjunct assistant clinical professor at the University of the Incarnate Word’s (UIW) Rosenberg School of Optometry.
Dr. Mueller is an ophthalmologist at Parkhurst NuVision.
Dr. Vanrachack is an optometrist at Parkhurst NuVision and an adjunct assistant clinical professor at UIW’s Rosenberg School of Optometry.
Dr. Davies is the chief of oculoplastic surgery for the US Airforce and Parkhurst NuVision.
1. Rootman J. Diseases of the Orbit: A Multidisciplinary Approach. Hagerstown: Lippincott Williams and Wilkins; 2003. 2. Phillips D. Epidemiology of Graves’ disease. In: Rapoport B, McLachlan SM, eds. Graves’ Disease. Endocrine Updates, vol 6. Boston: Springer; 2000. 3. Basic and Clinical Science Course 2015 - 2016. American Academy of Ophthalmology. 2015:43-60. 4. Maheshwari R, Weis E. Thyroid associated orbitopathy. Indian J Ophthalmol. 2012;60(2):87-93. 5. Wiersinga WM, Smit T, van der Gaag R, Koornneef L. Temporal relationship between onset of Graves’ ophthalmopathy and onset of thyroidal Graves’ disease. J Endocrinol Invest. 1988;11(8):615-9. 6. Bartalena L, Baldeschi L, Dickinson AJ, et al. Consensus statement of the European Group on Graves’ Orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008;18(3):333-46. 7. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726-38. 8. Wiersinga WM, Prummel MF. Pathogenesis of Graves’ ophthalmopathy—current understanding. J Clin Endocrinol Metabol. 2001;86(2):501-3. 9. Dolman PJ. Evaluating Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):229-48. 10. Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168-99. 11. Bartley GB. Evolution of classification systems for Graves’ ophthalmopathy. Ophthal Plast Reconstr Surg. 1995;11(4):229-37. 12. Klatsky SA, Manson PN. Thyroid disorders masquerading as aging changes. Ann Plast Surg. 1992;28(5):420-26. 13. Rizzuti AE, Dastgir G, Gutman J, et al. The role of orbital CT in thyroid eye disease. Invest Ophthalmol Vis Sci. 2012;53:1014. 14. Amino N, Yuasa T, Yabu Y, et al. Exophthalmos in autoimmune thyroid disease. J Clin Endocrinol Metab. 1980;51(6):1232-34. 15. Migliori ME, Gladstone GJ. Determination of the normal range of exophthalmometric values for black and white adults. Am J Ophthalmol. 1984;98(4):438-42. 16. Boddu N, Jumani M, Wadhwa V, et al. Not all orbitopathy is Graves’: discussion of cases and review of literature. Front Endocrinol (Lausanne). 2017;8:184. 17. Lacey B, Chang W, Rootman. Nonthyroid causes of extraocular muscle disease. J Surv Ophthalmol. 1999;44(3):187-213. 18. Dickinson AJ. Clinical manifestations. In: Wiersinga WM, Kahaly GJ, eds. Graves’ orbitopathy: A multidisciplinary approach—questions and answers. Basel: Karger; 2010:1-25. 19. Frueh BR. Why the NOSPECS classification of Graves’ eye disease should be abandoned, with suggestions for the characterization of this disease. Thyroid. 1992;2:85-88. 20. Dolman PJ, Rootman J. VISA classification for Graves orbitopathy. Ophthal Plast Reconstr Surg. 2006;22(5):319-24. 21. Boboridis K, Perros P. General management plan in Graves’ orbitopathy: a multidisciplinary approach. Basel:Karger; 2007:88-95. 22. Gerding MN, van derMeer JW, Broenink M, et al. Association of thyrotropin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2000;52:267-71. 23. Gould DJ, Roth FS, Soparkar CNS. The diagnosis and treatment of thyroid-associated ophthalmopathy. Aesth Plast Surg. 2012;36(3):638-48. 24. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338(2):73-78. 25. Kishazi E, Dor M, Eperon S, et al. Thyroid-associated orbitopathy and tears: a proteomics study. J Proteomics. 2018;170:110-6. 26. Sahlı E, Gündüz K. Thyroid-associated ophthalmopathy. Turk J Ophthalmol. 2017;47(2):94-105. 27. Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454-63. 28. Sisti E, Coco B, Menconi F, et al. Intravenous glucocorticoid therapy for Graves’ ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endocrinol. 2015;172(3):269-76. 29. Tanda ML, Bartalena L. Efficacy and safety of orbital radiotherapy for Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(11):3857-65. 30. Marcocci C, Bartalena L, Tanda ML, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86(8):3562-7. 31. Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16(8):2458-65. 32. Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348-54. 33. Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119(2):940-2. 34. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397-405. 35. Chen H, Mester T, Raychaudhuri N, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. w2014;99(9):E1635-40. 36. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-61. 37. Wiersinga WM, Kahaly GJ. Graves orbitopathy: A multidisciplinary approach. Basel: Karger; 2007. 38. Cawood TJ, Moriarty P, O’Farrelly C, O’Shea D. Smoking and thyroid-associated ophthalmopathy: A novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92(1):59-64. 39. Krassas GE, Wiersinga W. Smoking and autoimmune thyroid disease: The plot thickens. Eur J Endocrinol. 2006;154(6):777-80. |

