 In last months column, Dr. Joseph Sowka recounted his recent trip to
In last months column, Dr. Joseph Sowka recounted his recent trip to
The issue of chloramphenicol use is indeed unique. Over the last 40 years, our profession has seen the introductionand demiseof numerous topical antibiotics. In most of these instances, though, the antibiotics in question failed because of insufficient efficacy.
Chloramphenicol is one of few agents to be discontinued because of a perceived high risk of morbidity due to adverse reaction, namely aplastic anemia. Contrast this with drugs like sulfacetamide, tetracycline and norfloxacin, which simply proved inferior to more broad-spectrum antimicrobial agents.
Antibiotics may also fall out of favor because of local toxicity. Gentamicin and ciprofloxacin maintain reasonable efficacy against a wide range of microbial pathogens, even after 24 and 17 years of use, respectively; but, they are notorious for potential corneal epithelial disruption.1 In cases of excessive or prolonged use, medicamentosa can be particularly common.
Increased Resistance
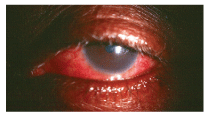
A toxic reaction to topical gentamycin. This patient was treated for a standard bacterial conjunctivitis for several days and returned complaining of increased discomfort, redness and ocular swelling.
The discussion of chloramphenicol brings to light another important consideration: bacterial resistance.
Resistance, the gradual decrease of an antibiotics efficacy against a pathogen, is caused by bacterial mutation and selection pressure, but it is fueled by inappropriate prescribing habits.2,3
Chloramphenicol still possesses a broad spectrum of activity against common pathogens, and one study suggests that resistance to chloramphenicol may have actually diminished over the last 20 years because of decreased prescribing rates.4-8
But, more commonly prescribed antibiotics have experienced the opposite effect. Recent reports show increased resistance to tobramycin and ofloxacin by some common gram-positive organisms, such as Staphylococcus aureus and Streptococcus pneumoniae.8,9
Most eye-care physicians in the
In light of this, it is difficult to understand the recent introduction of a topical azithromycin product, AzaSite (azithromycin ophthalmic solution 1%, Inspire). Azithromycin, despite its tremendous popularity within primary care and pediatric circles, is a comparatively older antibiotic; it was conceived in the 1980s and used widely in
Azithromycin is a macrolide antibiotic. It is closely related to erythromycin, but it has a much longer half-life and can be dosed with less frequency. Early reports demonstrated that azithromycin also had substantially lower MICs than erythromycin, especially in the case of certain gram-negative bacteria (e.g., Haemophilus influenzae, Neisseria gonorrhea and Chlamydia trachomatis).13 But now, azithromycin is at the center of a worldwide controversy.
Recent articles have expressed concern over the widespread systemic use of azithromycin and high levels of macrolide resistance in several bacterial species, most notably S. pneumoniae.14-16 In one study, researchers dosed 74 healthy patients with 500mg of azithromycin daily for three days and then examined bacterial flora from their nasopharyngeal carriages. The mean proportion of macrolide-resistant streptococci rose more than 60% from baseline just one day after therapy.17 These levels remained elevated for six months.17
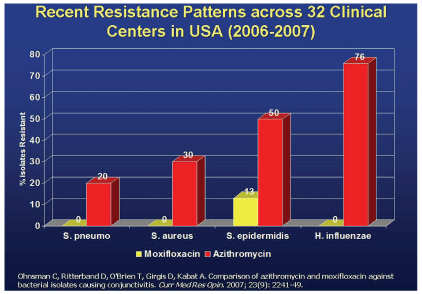
A study from the Bascom Palmer Eye Institute found significant resistance to azithrymycin.
Of course, systemic resistance does not always correlate directly with ocular resistancethe concentration of antibiotics achieved in tissues of the eye with topical agents may exceed the plasma levels used to calculate MICs.18 Such an argument may be true for concentration-dependent antibiotics like fluoroquinolones. But, azithromycin displays a time-dependent pharmacodynamic profile; increased concentration affords the drug little capacity to overcome macrolide-resistant bacterial strains.19
Research has shown that common ocular pathogens already display significant resistance to azithromycin. One study found high MICs for this drug against endophthalmitis isolates.20 Another, from the Bascom Palmer Eye Institute, suggests resistance rates as high as 50% (Staphylococcus epidermidis) or 76% (H. influenza), while a third demonstrated notable azithromycin resistance in ocular isolates, including S. aureus and Pseudomonas aeruginosa.21,22
Under identical conditions, these studies showed virtually no resistance among the same pathogens to moxifloxacin or gatifloxacin.21,22
Selecting an appropriate antibiotic requires consideration of solubility, tissue concentration, cost, dosing regimen and toxicity. Most importantly, select a drug that provides a broad spectrum of activity when employing empiric therapy for ocular infection and prophylaxis. In such situations today, scientific evidence supports the use of fourth-generation fluoroquinolones. Other options, no matter how attractive their dosing frequency or price may seem, have more potential for failure and put both doctor and patient at risk.
Dr. Kabat is a member of Alcons Speakers Alliance and the Board of Optometric Consultants for CYNACON/OCuSOFT. He has no direct financial interest in any of the products mentioned in this article.
1. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea 2006 Dec;25(10):1133-8.
2. Cunha BA. Antibiotic resistance. Med Clin North Am 2000 Nov;84(6):1407-29.
3. Barlow G, Nathwani D. Is antibiotic resistance a problem? A practical guide for hospital clinicians. Postgrad Med J 2005 Nov;81(961):680-92.
4. Usha K, Smitha S, Shah N, et al. Spectrum and the susceptibilities of microbial isolates in cases of congenital nasolacrimal duct obstruction. J AAPOS 2006 Oct;10(5):469-72.
5. Arantes TE, Cavalcanti RF, Diniz Mde F, et al. Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Arq Bras Oftalmol 2006 Jan-Feb;69(1):33-6.
6. Orden Martnez B, Martnez Ruiz R, Milln Prez R. Bacterial conjunctivitis: most prevalent pathogens and their antibiotic sensitivity. An Pediatr (Barc) 2004 Jul;61(1):32-6.
7. Egger SF, Ruckhofer J, Alzner E, et al. In vitro susceptibilities to topical antibiotics of bacteria isolated from the surface of clinically symptomatic eyes. Ophthalmic Res 2001 Mar-Apr;33(2):117-20.
8. Chalita MR, Hfling-Lima AL, Paranhos A Jr., et al. Shifting trends in in vitro antibiotic susceptibilities for common ocular isolates during a period of 15 years. Am J Ophthalmol 2004 Jan;137(1):43-51.
9. Kurokawa N, Hayashi K, Konishi M, et al. Increasing ofloxacin resistance of bacterial flora from conjunctival sac of preoperative ophthalmic patients in Japan. Jpn J Ophthalmol 2002 Sep-Oct;46(5):586-9.
10. Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol 2002 Apr;133(4):463-6.
11. Kowalski RP, Dhaliwal DK, Karenchak LM, et al. Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am J Ophthalmol 2003 Sep;136(3):500-5.
12. Ballow CH, Amsden GW. Azithromycin: the first azalide antibiotic. Ann Pharmacother 1992 Oct;26(10):1253-61.
13. Kanatani MS, Guglielmo BJ. The new macrolides. Azithromycin and clarithromycin. West J Med 1994 Jan;160(1):31-7.
14. Barkai G, Greenberg D, Givon-Lavi N, et al. Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis 2005 Jun;11(6):829-37.
15. Bergman M, Huikko S, Huovinen P, et al. Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother 2006 Nov;50(11):3646-50.
16. Kaplan EL, Cornaglia G. Persistent macrolide resistance among group A streptococci: the lack of accomplishment after 4 decades. Clin Infect Dis 2005 Sep;41(5):609-11.
17. Malhotra-Kumar S, Lammens C, Coenen S, et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double- blind, placebo-controlled study. Lancet 2007 Feb;369(9560):482-90.
18. Kowalski RP, Yates KA, Romanowski EG, et al. An ophthalmologist"s guide to understanding antibiotic susceptibility and minimum inhibitory concentration data. Ophthalmology 2005 Nov;112(11):1987.
19. Carbon C. Pharmacodynamics of macrolides, azalides, and streptogramins: effect on extracellular pathogens. Clin Infect Dis 1998 Jul;27(1):28-32.
20. Mather R, Kowalski RP, Whitcher JP. The in vitro susceptibility of bacterial endophthalmitis isolates to azithromycin. Paper presented at The Ocular Microbiology and Immunology Group (OMIG).
21. Ohnsman C, Ritterband D, OBrien T, Kabat A. Comparison of azithromycin and moxifloxacin against bacterial isolates causing conjunctivitis. Curr Med Res Opin 2007 Sep;23(9):2241-49.
22. Ficco CW, Blondeau JM. Antimicrobial efficacy of gatifloxacin compared with azithromycin against common ocular pathogens. Poster presented at the AAO.

