Psychoactive drugs are prescribed frequently for a variety of conditions that alter mood and emotional status. Whether it’s short-term use of an antidepressant to cope with the loss of a loved one or a lifelong reliance on an anti-schizophrenia drug to function normally in everyday life, approximately 46% of Americans will meet the criteria for a DSM-IV and -V (Diagnostic and Statistical Manual of Mental Disorders) disorder at some point in their lifetime.1,2 Furthermore, half of all lifetime cases are known to begin by age 14 and three quarters by age 24.1
Although many potential neurological complications exist, the eye is the second most common site to manifest drug toxicity, outshined only by the liver.4 As such, optometrists should follow individuals placed on these agents diligently. A standardized approach for monitoring ocular toxicities from these medications and others would be helpful. The astute clinician should be aware of these agents and their potential complications so that timely intervention can be started to prevent permanent sequelae.
Proposed ramifications of ocular toxicity include: ocular surface and ocular pigmentation complications, cataracts, accommodative interference, angle closure glaucoma, retinopathy and neuropathy, ocular motility disorders and impaired sensory perception.4 Two systemic considerations that, although not directly related to the eye, can have ocular consequences include increased risk of metabolic syndrome, specifically diabetes, and cerebrovascular events.
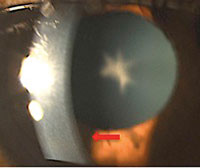 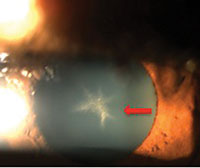 | |
| A 54-year-old black female with pertinent history of clozapine 100mg PO daily use for schizophrenia. No previous history of typical antipsychotic medication use. Her best-corrected visual acuity is 20/20 OU. Note the fine golden-brown dust-like deposits on the corneal endothelium, greatest inferiorly, and anterior subcapsular opacity in a stellate pattern in both eyes. No pigmentary retinopathy was noted. |
The Ocular Surface
Drug toxicity to the ocular surface can manifest as epithelial keratopathy, corneal edema and altered tear film quantity and quality.5 Timely and appropriate management of these complications can prevent permanent ocular surface damage and impaired vision. Early recognition can ensure the patient is able to continue the appropriate treatment for their underlying disorder.
Phenothiazines (typical antipsychotics), for example, can cause phototoxic lysis of the corneal endothelium.6 This is linked to total dosage of medication.5,6 This can lead to impaired endothelial pump function, causing severe corneal edema and consequent severe visual effects.6 Visual impairment is irreversible without prompt identification and cessation of the medication.
Chlorpromazine in particular, a member of the phenothiazine family, has been noted to cause corneal epithelial keratopathy.5 The manifestation seen with chlorpromazine use is noted to have a distinctive pattern of swirling lines or fine streaks in the epithelium, reminiscent of Plaquenil (Covis Pharmaceuticals) keratopathy.5 This presentation has been linked to high dosages (>2g/day) of the medication.5 The condition elicits minimal visual consequences and usually regresses with appropriate intervention, such as dose tapering.5
Psychiatric Medications
|
Psychiatric medications also can act to alter both the quantity and quality of tears. Medications that have anticholinergic effects, such as tricyclic antidepressants (TCAs), may cause decreased lacrimation.7,8 One can postulate that any medication with such effects would decrease the quantity of tear production. Although particularly bothersome in CL wearers or post-LASIK patients, appropriate use of artificial tears or prescription cyclosporine can be helpful in such cases.
Clozapine, an atypical antipsychotic medication, is commonly used as an alternative to traditional phenothiazines due to a comparative reduced risk of neurological side effects.9 However, clozapine exhibits anticholinergic effects by blocking muscarinic and nicotinic receptors, as well as the muscarinic-3 receptors in the conjunctiva and lacrimal gland.10 This leads to decreased mucous and aqueous secretion.10 Persistent tear film instability and dry eye syndrome can induce morphological and biomechanical changes at the cellular level, inevitably affecting the ocular surface and vision.11 Prompt lubrication and tear rehab agents such as cyclosporine are required to prevent desiccation.
Alterations of the tear film quality may also manifest as changes to the chemical components of the tears occur. Lithium, one of the oldest mood stabilizers, has been reported to cause eye irritation during the first few weeks of treatment.12 A potential etiology may be an increased sodium concentration in lacrimal secretion due to lithium’s effects on sodium-chloride cotransport proteins.12 The increased tear osmolarity may diminish with sustained use as a new equilibrium is established, thus explaining why the toxicity is only seen with medication initiation.
Follow The Pigment
Beyond their effects on the ocular surface, both chlorpromazine and clozapine have been linked to ocular pigmentation; however, the former, a typical antipsychotic, is more likely to cause pigment changes compared with clozapine.4,13,14 Ocular pigmentation secondary to drug toxicity can be divided into two categories: pigmentation of the skin, conjunctiva, cornea or lens and pigmentation retinopathy.13,14 The first may cause minimal to no changes to vision, while the latter may lead to irreversible degenerative retinopathy.
Chlorpromazine is known to accumulate in the skin of the eyelid, conjunctiva, posterior corneal stroma, lens and uveal tract.4,13,14 As the compound is phototoxic, it has been postulated that photosensitization of the tissue proteins occurs in areas of increased sun exposure after accumulation of the drug in these tissues.13,14 Protective sun wear and reduced sun exposure while on the medication is recommended.
Although these pigment changes are not as common with the use of clozapine, there have been a handful of cases with a similar pathology.14 Although newer, safer alternatives are present in comparison with conventional treatments, clinicians should continue to be aware of potential, less common side effects that may still exist.
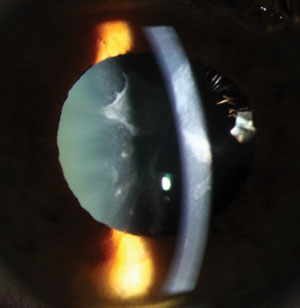 | |
| Cataracts secondary to medication are usually asymmetric, perhaps due to unequal deposition of medication in the crystalline lens. Photo: Christine W. Sindt, OD. |
Lenticular Opacities and Cataracts
Cataracts that develop through the natural aging process most often cause bilateral clouding of the crystalline lens; however, cataracts secondary to medication use are rarely bilateral and usually asymmetric.15 This may be due to unequal deposition of a foreign medication in the crystalline lens. Additionally, as certain antipsychotics—namely the atypical variety—can cause metabolic syndrome, hyperglycemic status in these patients can also lead to early diabetic cataract formation.15 Interestingly, lenticular opacities are often found in conjunction with corneal opacities, yet not all represent true cataractous changes.15
Phenothiazines such as chlorpromazine and thioridazine are the most common antipsychotic agents that lead to lenticular opacities.4,7,8 As described above, posterior corneal pigmentation can be related to anterior subcapsular pigment, which may show a link to alterations in the aqueous humor.15,16
Although phototoxicity may be a potential mechanism of action, an alternate explanation may be lens discoloration. Endogenous melanin in the eye could be trapping free radicals produced by psychotropic medications, which may show up as lens discoloration in certain cases.15,16 These opacities may not reverse with cessation of the causative agent, yet any resultant visual disturbance will vary based on the duration of exposure and degree of damage.15,16
Accommodative Interference
Changes to accommodative status in patients using psychiatric medications occur mainly due to the drugs’ anticholinergic effects. The common effects include mydriasis and cycloplegia due to combinations of sympathetic stimulation; TCAs, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs) and norepinephrine reuptake inhibitors (NRIs) have been shown to cause both mydriasis and cycloplegia in the acute phase of treatment.17
Mydriasis—dilation of the pupil due to stimulation of alpha receptors located on the radial muscle of the iris—may cause non-severe and transient visual changes. Cycloplegia, however, has a paretic effect on the ciliary muscle and may lead to blurred vision, mainly at near. Management of accommodative disability may require an appropriate spectacle prescription until the condition improves. Moreover, it can be assumed that any psychotropic medication with strong anticholinergic actions, antiadrenergic actions or both can cause mydriasis and cycloplegia with similar effects.
Along with an anticholinergic effect, SSRIs have multiple actions on the central and peripheral nervous system, including muscles of the iris that cause pupil dilation and constriction. An alternative mechanism of action for mydriasis by SSRIs may be stimulation of 5-HT7 receptors.18 These receptors are located in the iris sphincter and, when stimulated, cause a passive mydriasis.18 They are likely also involved in increased intraocular pressure and SSRI-related glaucoma.
| Table 1. Psychiatric Medications and their Ocular Complications8 | ||
| Ocular Complication | Medication | |
| Refractive error | Topamax | |
| Increased intraocular pressure | Topamax, antidepressants (TCAs/SSRIs/SNRIs) | |
| Accommodative interference | Topamax, antidepressants (TCAs/SSRIs) | |
| Ocular motor disturbances | Anticonvulsants, Topamax, anxiolytics, lithium | |
| Oculogyric crisis | Typical antipsychotics, atypical antipsychotics, Topamax, anticonvulsants | |
| Tear film changes | Lithium, antidepressants | |
| Corneal or lenticular opacities | Typical antipsychotics, rarely atypical antipychotics | |
| Pigmentary retinopathy | Typical antipsychotics, rarely anticonvulsants and anxiolytics | |
Topamax (topiramate, Janssen Pharmaceuticals), for example, is an anticonvulsant that may cause blurred vision secondary to changes caused by idiosyncratic swelling of the ciliary body, leading to a refractive change and myopic shift in prescription.8,19 The World Health Organization has identified at least 13 adverse ocular side effects of Topamax, classified as “certain,” “probable/likely” or “possible.”20 Abnormal vision and acute myopia (up to -8.75D) were categorized as potential side effects of Topamax use.20 Associated symptoms likely occur within the first month of treatment.20 Also note that this medication is often used off-label for migraine relief.
IOP and Angle Closure Glaucoma
Risk factors for acute angle closure glaucoma include: race, increased age, narrow anterior chamber angle, shallow anterior chamber depth, hyperopia, nanophthalmos, previous angle closure of fellow eye, family history, female sex and use of any substance that causes pupillary dilation.21 Selecting the appropriate treatment for acute angle closure requires a firm understanding of the mechanism in play. Ask yourself: is the condition a result of an anatomically narrow angle further aggravated into pupil block by a medication causing mydriasis and cycloplegia? Or is it due to choroidal effusion, ciliary body swelling and forward displacement of the iris-lens structures, thus crowding the angle and causing angle closure without pupil block? These two situations would require individualized treatment plans.
TCAs, SSRIs, SNRIs, typical antipsychotics and theoretically any psychiatric medication having anticholinergic effects can cause an acute angle closure with pupil block in a patient with anatomically narrow angles.8 The mechanism in such a case would be drug-induced mydriasis in an already crowded angle, either active or passive, depending on the drug. Thus, these drugs should be prescribed cautiously in patients with narrow angles. Furthermore, this builds a case for including a comprehensive medication review into routine eye exams and appropriate correspondence with primary care physicians for at-risk patients.
The exact mechanism behind secondary angle closure as related to Topamax remains unclear, although suprachoroidal effusion is likely involved.19,22 In addition, the sulfa-based molecule could potentially produce an allergic reaction, causing swelling of the lens and ciliary body.19,22 Here, aggressive cycloplegia is the treatment of choice, as opposed to the traditional line of thinking with an acute angle closure in which mydriasis/cycloplegia would further exacerbate the condition.22 The perceptive clinician should be able to distinguish between the two and select appropriate treatment. Recently, Topamax has been linked to visual field defects independent of increased intraocular pressure or angle closure attack, causing eye care providers to monitor patients accordingly.23
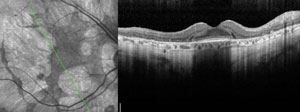 | ||
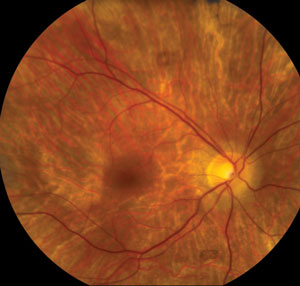 | ||
| Clinical signs and symptoms of thioridazine toxicity include decreased visual acuity, visual field defects and retinal pigment epithelial disturbances. There is selective uptake of thioridazine in pigmented uveal tissue and the retinal pigment epithelium, which results in toxicity. Photos: Christine W. Sindt, OD. | ||
As most of these ocular complications arise in the first 49 days of treatment, and even after the first dose, it is critical for clinicians encountering patients on this treatment modality to establish a length of time on treatment.20,23 Appropriate correspondence with the neurologist is warranted, as cessation of the medication, with or without initiation of other medical intervention, halts the condition and can save vision.20,23 A traditional laser peripheral iridotomy (LPI) would not be beneficial in this case, as the mechanism of action differs from a typical acute angle closure attack.22 Also, patients taking Topamax without ocular complications warrant a yearly evaluation.
SSRIs and SNRIs are also linked to increased IOP via a different mechanism involving the 5-HT7, 5-HT2A and 5-HT2C receptors located in the iris-ciliary body complex.18 These receptors cause an increase in flow to the ciliary body, thereby increasing aqueous humor production and IOP.18 Coupling this with the active and passive mydriasis caused by these or other medications, angle closure with pupil block can occur concomitantly.18
Retinopathy and Optic Nerve Involvement
Because the eye is developmentally and anatomically an extension of the brain and the retina is on that continuum, it can be affected by medications that have psychotropic effects. Also, this thin and fragile tissue can act as a reservoir for deposition of the drug reaching the eye through its vascular supply.8 As such, drug toxicity can affect both the retinal pigment epithelium (RPE) and the neurosensory retina (mainly, rods and cones).4,8 Deposited medication in either the RPE or sensory retina is not easily cleared and can lead to potential damage.
Ocular pigmentation caused by phenothiazines, such as thioridazine and chlorpromazine, may take place in the retina. Thioridazine’s risk is a function of large daily doses (>800 mg/day), while the risk associated with chlorpromazine is related to daily dose and duration of use.4,8,24 Pigment has been shown to deposit from the peripheral to central retina.24 Researchers postulate that phototoxic stress causes peripheral vision loss, nyctalopia, permanent vision loss and complete blindness as damage progresses.23 Early detection and intervention can prevent permanent visual consequences.
Researchers have hypothesized a possible link to retinal toxicity and melanin binding, as the RPE is rich in melanin. However, studies show that binding affinity of medications to ocular melanin is not predictive of drug toxicity.25
Retinal toxicity has been described in conjunction with benzodiazepines in a small number of cases.26 Clonazepam, an anticonvulsant and anxiolytic, has been linked to a ‘white-dot-like’ retinopathy that can be seen using ophthalmoscopy and confirmed with fundus autofluorescence.25 Optic nerve involvement in the form of optic disc swelling causing blurred vision has been documented with therapeutic doses of lithium.27 Resolution of this pathology has been seen with cessation of the medication. Regardless of exact cause, a need for yearly screening for retinopathy or papillopathy in patients taking high-risk medications is indicated. FAF screening may also be of value.
Ocular Motility Disorders
Oculogyric crisis caused by dystonia is due to involuntary contractions of various extraocular muscles. This may cause any variant of gaze paralysis, can be painful and even life-threatening if systemic muscles become involved.5 The etiology is complex and likely involves dopamine receptor blockage along with other causes.6,8 Antipsychotics, carbamazepine, Topamax and SSRIs all have been associated with oculogyric crisis.4,8 Treatment of ocular dystonia includes anticholinergic agents (i.e., intramuscular benzatropine) and antihistamines (i.e., diphenhydramine) and can be rapidly effective in reversing symptoms.4,6,8
Benzodiazepines have been linked to dysfunction of saccades and smooth-pursuits and nystagmus.28,29 Research has shown a correlation between downbeat nystagmus and lithium use, which can be reversed with termination of the medication; rarely, it may persist after discontinuation.30 Diplopia, oscillopsia, gaze-evoked nystagmus, gaze palsies, downbeat nystagmus and periodic alternating nystagmus have been associated with carbamazepine use.31 Lamotrigine (Lamictal, GlaxoSmithKline), an anticonvulsant, can cause a downbeat nystagmus, diplopia and interference with eye movements in overdoses or in combination with carbamazepam.4,8 Finally, Topamax can induce nystagmus in high doses, although it is not well understood if the cause is idiosyncratic.4,8 Given the vast array of medications that can cause nystagmus and motility disorders, it is important to consider psychotropic medications in the differentials when evaluating a patient with ocular dysmotility.
Impaired Sensory Perception
Changes to color vision and contrast sensitivity can be noted with use of psychiatric medications. Central and paracentral color vision decrease has been clearly demonstrated with use of carbamazepine; however, the effect is subclinical and requires only monitoring.32 Contrast sensitivity function can be reduced in patients taking carbamazepine and benzodiazepines.8 Changes to sensory perception indicate a need for monitoring visual function above and beyond visual acuity. Regular color vision and contrast sensitivity testing should occur in patients using these medications.
Metabolic and Cerebrovascular Implications
Although the literature indicates that newer antipsychotics have safer toxicology profiles compared with typical antipsychotics, newer medications may still cause metabolic syndrome. Specifically, impaired glucose metabolism, exacerbation of existing Type 1 and 2 diabetes, induction of Type 2 diabetes and diabetic ketoacidosis have all been linked to treatment with antipsychotic medications.15,33 Clinicians should routinely screen for newly forming diabetic retinopathy in patients taking chlorpromazine, clozapine and olanzapine.15,33
Lastly, there has been much discussion in the literature regarding the increased risk of cerebrovascular events in elderly patients with dementia treated with antipsychotic medications.33 Typical antipsychotic agents have been associated with higher risk of cerebrovascular accidents.34 Statistically significant risk has not been noted with atypical medications and seems to dissipate with discontinuation.34
Dr. Minhas is a member of the clinical faculty at Salus University in Philadelphia, where she works with both the traditional optometry program and the Scholars Optometry Program.
A special thank you to Andrew Gurwood, OD, for his guidance and mentorship.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 June;62(6):593-602.2. American Psychiatric Association DSM-5 Development. 2014. Accessed November 22, 2015. www.dsm5.org/about/Pages/DSMVOverview.aspx.
3. Greenshaw, AJ. Neurotransmitter interactions in psychotropic drug action: beyond dopamine and serotonin. J Psychiatry Neurosci. 2003 Jul; 28(4):247-250.
4. Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31(2):127-41.
5. Johnson AW, Buffaloe WJ. Chlorpromazine epithelial keratopathy. Arch Ophthalmol. 1966;76(5):664-7.
6. Hansen TE, Casey DE, Hoffman WF. Neuroleptic intolerance. Schizophr Bull. 1997;23(4):567-82.
7. Malone DA Jr, Camara EG, Krug JH Jr. Ophthalmologic effects of psychotropic medications. Psychosomatics. 1992;33(3):271-7.
8. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents. CNS Drugs. 2010;24(6):501-26.
9. Claghorn J, Honigfeld G, Abuzzahab FS Sr, et al. The risks and benefits of clozapine versus chlorpromazine. Journal of Clinical Psychopharmacology. 1987;7(6);377-84.
10. Pramanik T, Ghising R. Salivation induced better lacrimal gland function in dry eyes. Nepal Med Coll J. 2009;11:258-60.
11. Ceylan E, Ozer MD, Yilmaz YC, et al. The ocular surface side effects of an anti-psychotic drug, clozapine. Cutan Ocul Toxicol. 2015 Apr 8:1-5. [Epub ahead of print].
12. Lauf PK, Chimote AA, Adragna NC. Lithium fluxes indicate presence of Na-Cl cotransport (NCC) in human lens epithelial cells. Cell Physiol Biochem. 2008;21(5-6):335-46.
13. Greiner AC, Berry K. Skin pigmentation and corneal and lens opacities with prolonged chlorpromazine therapy. Canad Med Ass J. 1964;90:663-5.
14. Borovik AM, Bosch MM, Watson SL. Ocular pigmentation associated with chlozapine. Med J Aust. 2009 Feb;190(4):210-1.
15. Shahzad S, Suleman MI, Shahab H, et al. Cataract occurrence with antisychotic drugs. Psychosomatics. 2002;43(5):354-9.
16. Oshika T. Ocular adverse effects of neuropsychiatric agents: incidence and management. Drug Saf. 1995;12(4):256-63.
17. Lieberman E, Stoudemire A. Use of tricyclic antidepressants in patients with glaucoma: assessment and appropriate precautions. Psychosomatics. 1987;28(3):145-8.
18. Costaglogiola C, Parmeggiani F, Sebastiani A. SSRIs and intraocular pressure modifications: evidence, therapeutic implications and possible mechanisms. CNS Drugs. 2004;18(8):475-84.
19. Rhee DJ, Goldberg MJ, Parrish RK. Bilateral angle-closure glaucoma and ciliary body swelling from topiramate. Arch Ophthalmol. 2001;119(11):1721-3.
20. Fraunfelder FW, Fraunfelder FT. Adverse ocular drug reactions recently identified by the National Registry of Drug-Induced Ocular Side Effects. Ohthalmology. 2004;111(7):1275-9.
21. Tripathi RC, Tripathi BJ, Haggerty C. Drug-induced glaucomas: mechanism and management. Drug Saf. 2003;26(11):749-67.
22. Sankar PS, Pasquale LR, Grosskreutz CL. Uveal effusion and secondary angle-closure glaucoma associated with topiramate use. Arch Ophthalmol. 2001;119:1210-1.
23. U.S. Food and Drug Administration. (n.d.). Topomax (topiramate) tablets and sprinkle capsules. Accessed November 24, 2015. www.fda.gov/Safety/MedWatch/SafetyInformation/ucm195797.htm.
24. Siddall JR. Ocular toxic changes associated with chlorpromazine and thioridizine. Can J Ophthalmol. 1966;1:190-8.
25. Leblanc B. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regulatory Tox and Pharmacology. 1998;28(2):124-132.
26. Mateo J. Value of fundus autofluorescence imaging in a rare case of clonazepam associated retinopathy. Acta Ophthalmologica. 2012;90(s249).
27. Lobo A, Pilek E, Stokes PE. Papilledema following therapeutic dosages of lithium carbonate. J Nerv Ment Dis. 1978;166:526-9.
28. Bittencourt PRM, Wade P, Smith AT, Richens A. Benzodiazepines impair smooth pursuit eye movements. Br J Clin Pharmacol. 1983;15:259-62.
29. Rucker JC. Current treatment of nystagmus. Curr Treat Options Neurol. 2005;7(1):69-77.
30. Williams DP, Troost BT, Rogers J. Lithium-induced downbeat nystagmus. Arch Neurol. 1983;40:754-5.
31. Chrousos GA, Cowdry R, Schuelein M, et al. Two cases of downbeat nystagmus and oscillopsia associated with carbamazepine. Am J Ophthalmol. 1987;103:221-4.
32. Verrotti A, Lobefalo L, Priolo T, et al. Color vision in epileptic adolescents treated with valproate and carbamazepine. Seizure. 2004;13:411-7.
33. Haddad PM, Dursun SM. Neurological complications of psychiatric drugs: clinical features and management. Human Psychopharmacology. 2008;23:15-26.
34. Laredo L, Vargas E, Blasco AJ, et al. Risk of cerebrovascular accident associated with use of antipyschotics: population-based case-control study. J Am Geriatr Soc. 2011;59(7):1182-7.

