Statins are a class of lipid-altering drugs that revolutionized hypercholesterolemia treatment and aid in primary and secondary cardiovascular disease (CVD) prevention. An estimated 38.6 million Americans were on a statin regimen in 2011 and 2012, a nearly 38% increase from 24 million in 2003 and 2004.1 This rise in use makes statins the most commonly prescribed class of cholesterol-lowering drug. Due to this widespread use, it’s vital optometrists understand the possible effects they can have on patients’ eyes.
Although many studies into statins and ocular conditions show conflicting results or are of statistical insignificance, some results suggest statins could potentially mitigate various ocular conditions.
Here, we explain how statins affect different patients’ eyes—for better or for worse, and how the OD can oversee these patients and their ocular and visual health.
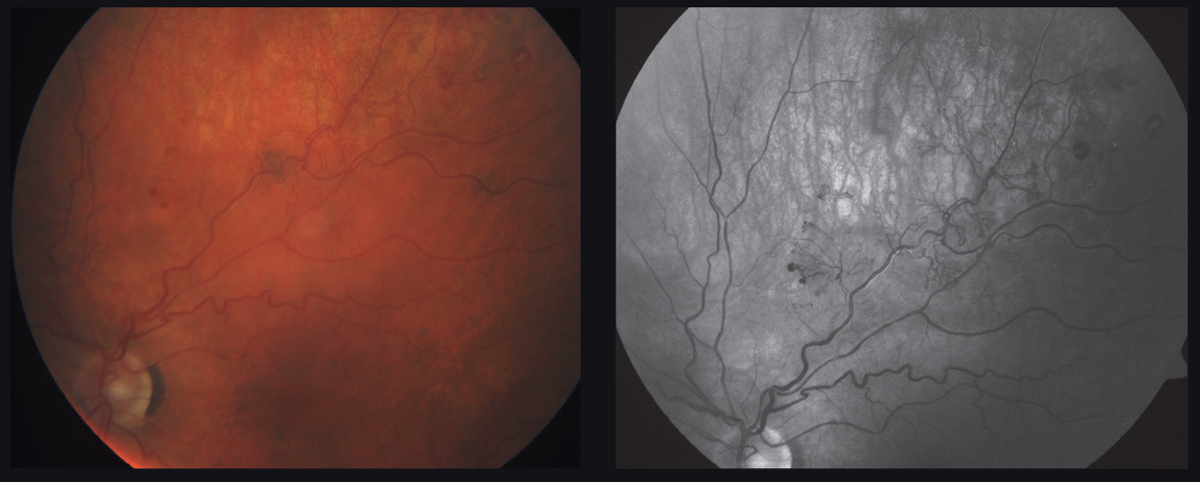 |
| This 61-year-old patient’s left eye has an ischemic branch retinal vein occlusion with evidence of frank neovascular edema. Click image to enlarge. |
Mechanism of Action
Statins are a potent drug for lowering low-density lipoprotein (LDL) cholesterol in the treatment of hypercholesterolemia and mixed hyperlipidemia. Through inhibition and regression of coronary atherosclerosis, anti-inflammatory properties and atherosclerotic plaque stabilization, statins decrease rates of CVD and mortality.1
Available statins include lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, rosuvastatin and pitavastatin.2,3 Statins’ beneficial effects result from their capacity to reduce cholesterol biosynthesis and modulate lipid metabolism from inhibition upon 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in the liver. HMG-CoA reductase is the enzyme that converts HMG-CoA into mevalonic acid, a cholesterol precursor.3 Statins are HMG-CoA inhibitors that alter the conformation of the enzyme when they bind to its active site, preventing HMG-CoA reductase from attaining a functional structure. The reduction of cholesterol in hepatocytes leads to the increase of hepatic LDL receptors, ultimately reducing circulating LDLs and its precursors.
Statins also impart a modest benefit in concentrations of triglycerides, high-density lipoproteins (HDL) and very low-density lipoproteins (VLDL).2 Moreover, statins exert pleiotropic effects such as improvement of endothelial function, enhancing the stability of atherosclerotic plaques, decreasing oxidative stress and inflammation, and inhibiting thrombogenic response.4
Adverse Effects
Statins are generally well tolerated, and adverse reactions occur less frequently than with other classes of lipid-lowering agents.2,3 Liver and muscle toxicity are the most notable adverse effects of statins.2,3 Although studies have reported conflicting results, statins could have effects on glucose metabolism in non-diabetic patients or affect glycemic control of diabetic patients.2,3
Intensive statin therapy may increase the risk of developing diabetes; however, the benefits of statins on cardiovascular events and mortality outweigh the risks. Patients with liver failure, renal insufficiency, hypothyroidism, advanced age and serious infections are more prone to experiencing such adverse reactions.2,3
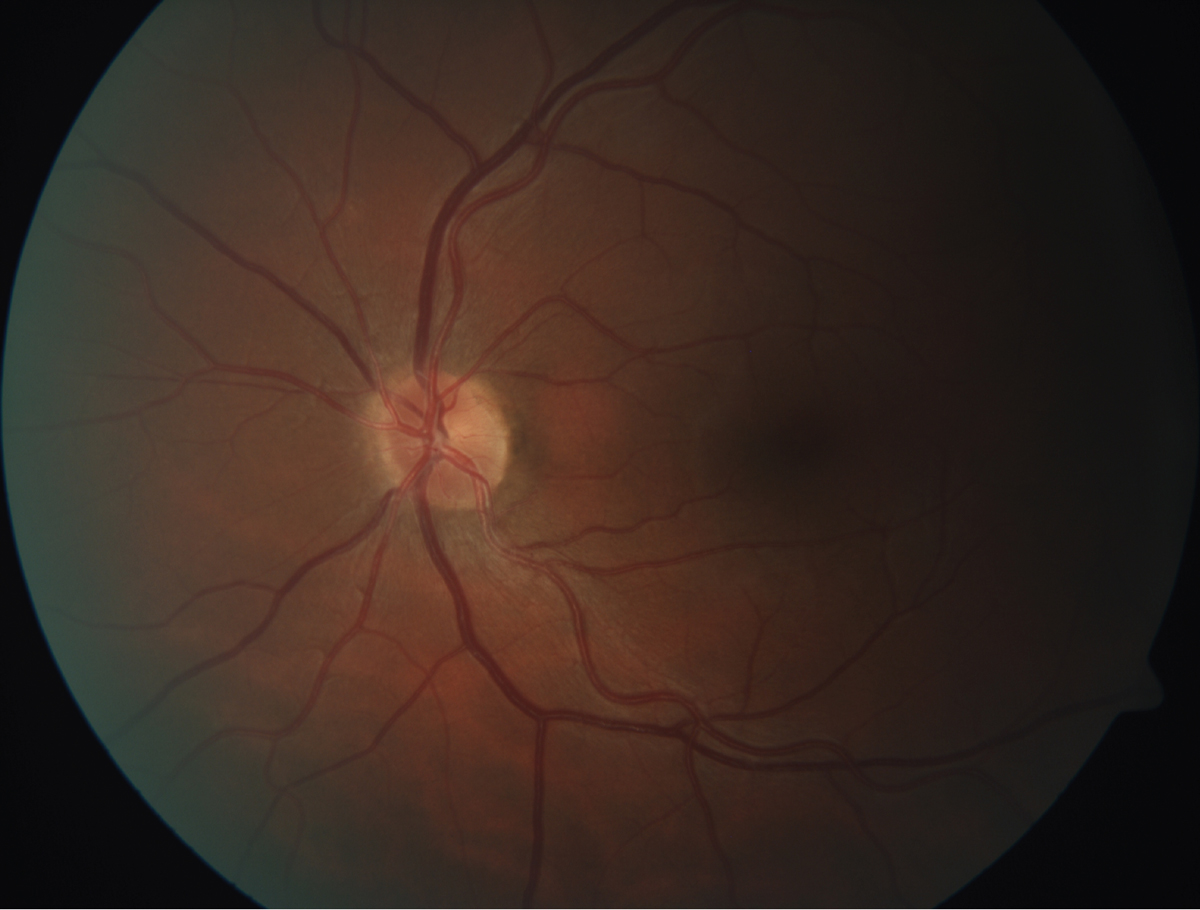 |
| This fundus image shows retinal arteriosclerosis in a 45-year-old patient with a total cholesterol of 304, triglycerides of 1,290 and LDL of 129. Click image to enlarge. |
The Anterior Segment
Adverse effects here range from exacerbation of dry eye to myopathy or possible accelerated cataract development, but positive associations exist as well.
Ocular surface. The Blue Mountains Eye Study III survey found that oral statins were associated with an increase in moderate to severe dry eye symptoms, possibly due to the disruption of essential cholesterol synthesis for meibum lipid homeostasis in the meibomian glands.5 Interestingly, a prospective pilot study with 10 dry eye and blepharitis patients found that topical atorvastatin may be a potential therapy, as demonstrated by improved tear film break up time, blepharitis score and bulbar conjunctival injection.6 While the mechanism is largely unknown, it may be due to the anti-inflammatory pleotropic effects; larger clinical studies are required to establish the efficacy and safety of topical statin use.
Orbit. Myopathies are a rare adverse reaction to statin therapy. Retrospective case reports suggest inflammation of the extraocular or levator muscles as a possible etiology; however, other patient risk factors such as advanced age, hypertension, diabetes and other comorbid cardiovascular conditions cannot be entirely ruled out. Of note, cases of ptosis, blepharoptosis and external ophthalmoplegia associated with statin usage have completely resolved upon discontinuation of statin use.7-9
The anti-inflammatory effects of statins may be beneficial in the area of thyroid eye disease. A recent report shows that oral statins may have the potential to decrease the conversion of Graves’ disease to thyroid-associated orbitopathy by 40%.10,11 A 2018 study of 30 patients shows that statin users tend to have a reduced number of orbital decompressions and strabismus surgeries compared with non-statin users.11
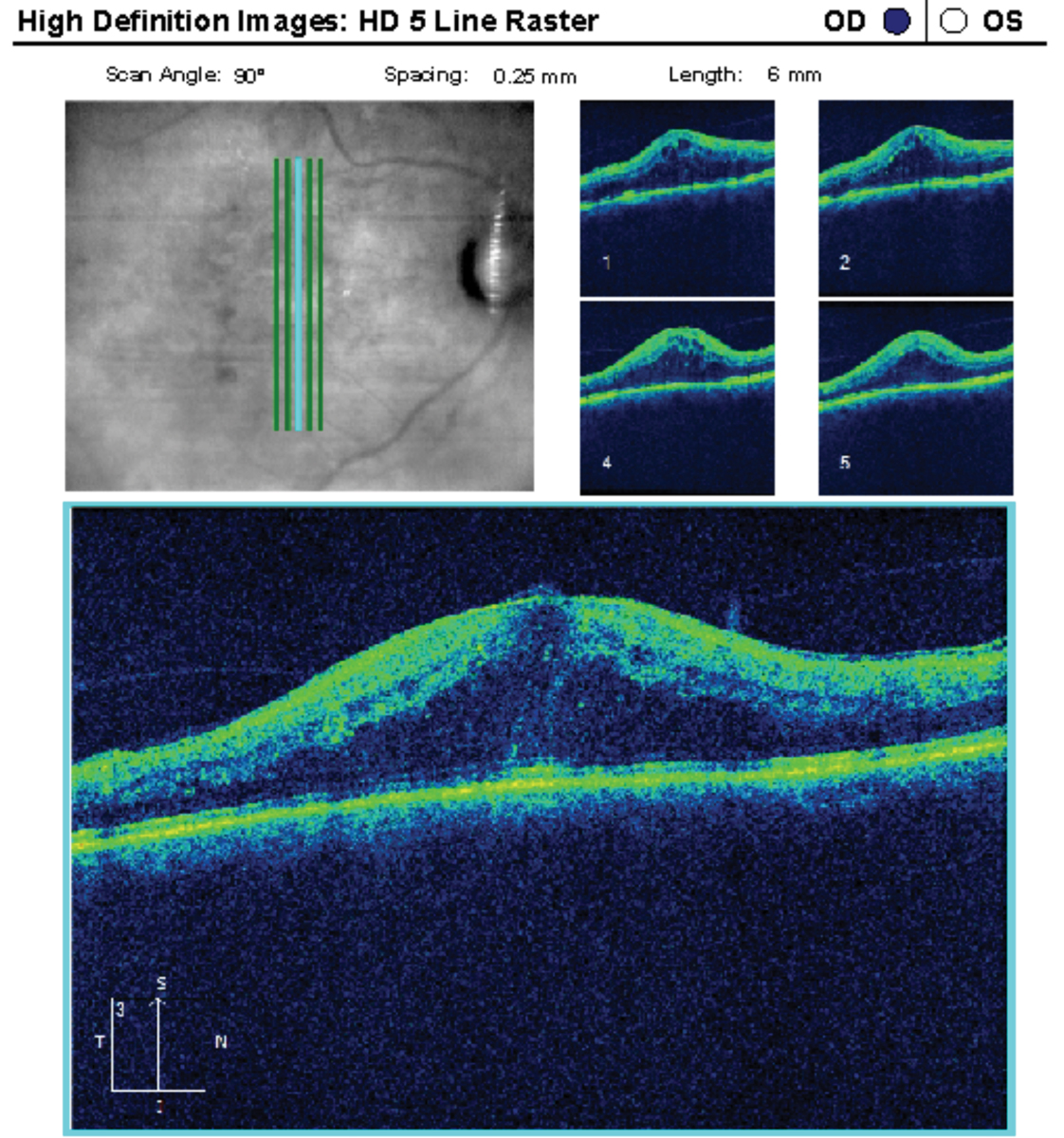 |
This 64-year-old patient had a history of poorly controlled insulin-dependent diabetes with significant DME in the right eye. She underwent two aflibercept injections previously. Some research shows statins may also reduce VEGF. Click image to enlarge. |
Ocular inflammation/uveitis. Statins may be beneficial in ocular inflammatory diseases by stabilizing the blood-ocular and blood-retinal barriers (BRB) as well as endothelial cells.12 Research also shows statins can reduce levels of key inflammatory markers, such as interleukins 6 and 8, TNF-alpha, and C-reactive protein.12,13 Research from 2015 found statin users were 48% less likely to develop uveitis than non-statin users.13
Cataracts. Studies on statin use and cataract development have yielded inconsistent and conflicting results. Some reports have found an increased risk of cataracts from statin use, while others showed no association or even a protective effect.14-16 One possible mechanism may be statins’ bidirectional effects on oxidation processes, including a possible mitochondrial effect that may increase the risk of cataracts.14 High cholesterol is required to maintain transparency of the crystalline lens; some researchers hypothesize that the inhibition of cholesterol biosynthesis by statin medications could prevent proper epithelial cell development within the crystalline lens, increasing the risk of cataract development.14
In contrast, a meta-analysis shows a clinically relevant protective effect of statins in preventing cataract formation, with a more pronounced effect in younger patients.16
Although the exact mechanism is unknown, statins may help to prevent cataracts through its pleotropic effects and decreasing by LDLs. In 2017, investigators published research on more than 313,200 cataract cases and concluded that no clear evidence shows that statin use increases the risk of cataract development.15
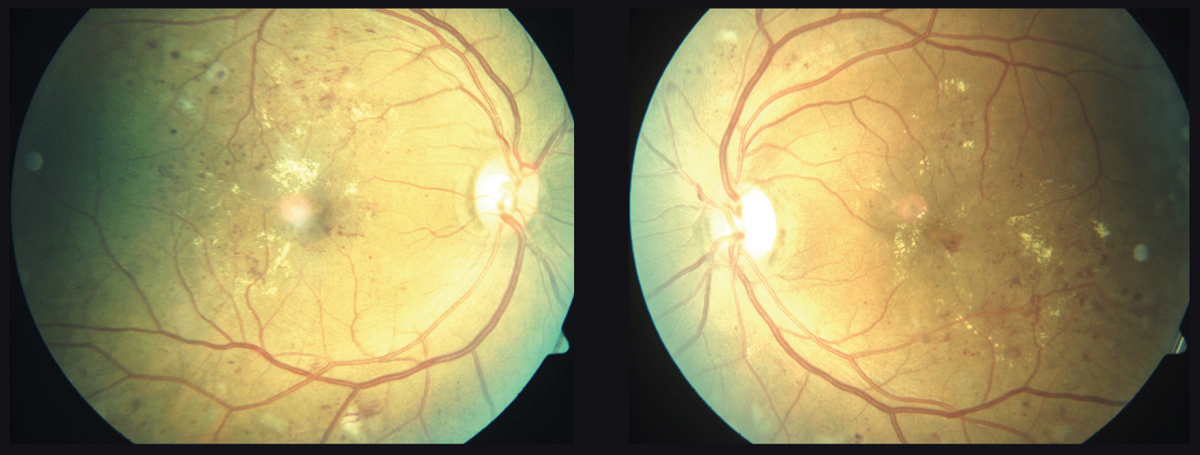 |
| This 55-year-old patient with diabetes developed significant bilateral DME over six months. Some patients who take statins are less likely to develop DME. Click image to enlarge. |
Posterior Segment
Could statins be protective against glaucoma, diabetic retinopathy or AMD? The jury is still out but some studies are encouraging.
Glaucoma/intraocular pressure. The current treatment of open-angle glaucoma (OAG) is aimed primarily towards intraocular pressure (IOP) reduction, although clinical challenges often arise when OAG progresses despite adequate IOP control. The search for other treatments, such a statins, is a rising area of interest amongst researchers.
Literature on statins and glaucoma are conflicting in nature. While some studies do not support statins’ protective role against OAG, recent research endorses their protective role.17-22 A large observational study of 136,782 participants reported that higher serum cholesterol levels were associated with a higher risk of primary OAG; five or more years of statin use was associated with a 21% lower risk, and use for 10 years or more had a 40% lower risk.20 Other studies have found that long-term use of statins is associated with a reduced risk of OAG (independent of the IOP); however, the results did not reach statistical significance.21
Researchers have proposed a few hypotheses to explain the mechanism of how statins reduce the risk of OAG. Statins increase aqueous outflow through the upregulation of endothelial nitric oxide synthase resulting in vasodilation and increased retinal and choroidal blood flow, leading to a reduction in IOP.20,22 They also inhibit rho-kinase activity, which may increase aqueous outflow and reduce IOP.20,22 The improved retinal and choroidal perfusion may benefit the health of the optic nerve and the nerve fiber layer. There are also potential neuroprotective effects that may protect retinal ganglion cells.20,22 Further studies are warranted to confirm statins’ potential benefit in OAG management.
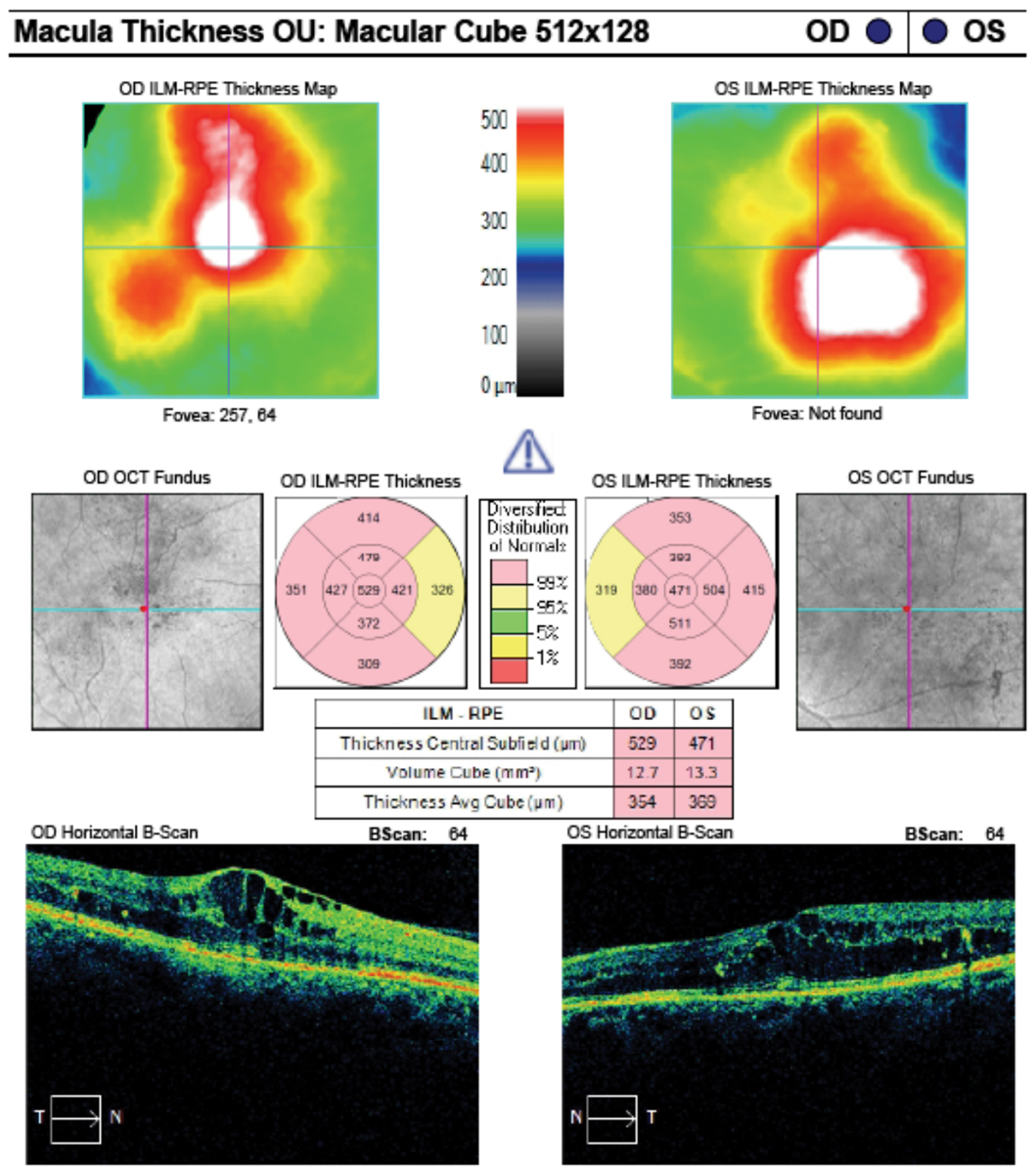 |
| These macular cube analysis images of the same patient from the previous page shows clinically significant macular edema in both eyes. Click image to enlarge. |
Diabetic retinopathy. Endothelial dysfunction and inflammatory processes lead to the development of diabetic retinopathy (DR). Statins have vasoprotective actions and are able to penetrate the BRB, reducing endothelial cell dysfunction and ultimately protecting against the sight threatening complications of DR.23-25 By reducing reactive oxygen species, increasing nitric oxide levels and increasing the number of endothelial progenitor cells, statins have the ability to improve vascular resistance, blood flow velocity and retinal perfusion thereby sustaining the stability of the BRB.26,27
In a study that looked at patients with Type 2 diabetes and dyslipidemia, those taking statins had a significantly lower rate of DR, nonproliferative DR, proliferative DR (PDR), vitreous hemorrhage, tractional retinal detachment and diabetic macular edema (DME) than the non-statin group.28 The study also indicted that statin users also had lower rates of ophthalmic interventions such as retinal laser treatment, intravitreal injection and vitrectomy.28 Moreover, these researchers reported a decreased risk in DR progression with the use of higher doses and longer duration of statin therapy.28
Two large clinical studies show fenofibrate has beneficial effects against DR progression, independent of its lipid-modifying action in Type 2 diabetics. Although fenofibrate is not classified as a statin medication, it can reduce the frequency of PDR by 30% and the need for laser treatment for DME by 31%.29 Additionally, one study also noted that the combination of fenofibrate with simvastatin synergistically reduced DR progression rate by 40%, compared with simvastatin treatment alone.29,30
Diabetic macular edema. Hypercholesterolemia is regarded as a strong risk factor for endothelial dysfunction, which contributes to the development of DME.31,32 In univariant analysis, the total cholesterol and LDL were significantly higher in patients with clinically significant macular edema and were a risk factor for retinal hard exudate formation.33-36
Results from a retrospective study show a noteworthy relationship between serum triglycerides and DME, which corresponds to the findings of the Fenofibrate Intervention and Event Lowering in Diabetes study. Additionally, a recent meta-analysis found that statins protected against the development of DME and progression of DR in patients with Type 2 diabetes.31,36,37 Large and long-term randomized controlled prospective studies are necessary to obtain a more complete assessment of the effects of statin therapy on DR and DME.
Vitrectomy for proliferative diabetic retinopathy. PDR is characterized by retinal neovascularization, contractile scar tissue formation, vitreous hemorrhage, tractional retinal detachment, and neovascular glaucoma. In randomized clinical trials, the use of simvastatin decelerated DR progression and visual acuity loss which may be attributed to the medication’s ability to suppress expression and secretion of potent vasoactive, proinflammatory and tissue remodeling factors.30,38-41
Similarly, a prospective observational study found that diabetic vitrectomy patients with preoperative statin treatment (mainly receiving lipophilic simvastatin or atorvastatin) had a better one-month best-corrected visual acuity improvement and a lower frequency of postoperative complications than those without statin treatment.42 These studies indicate that statins, particularly simvastatin, appear to contribute to a lower incidence of complications after diabetic vitrectomy.
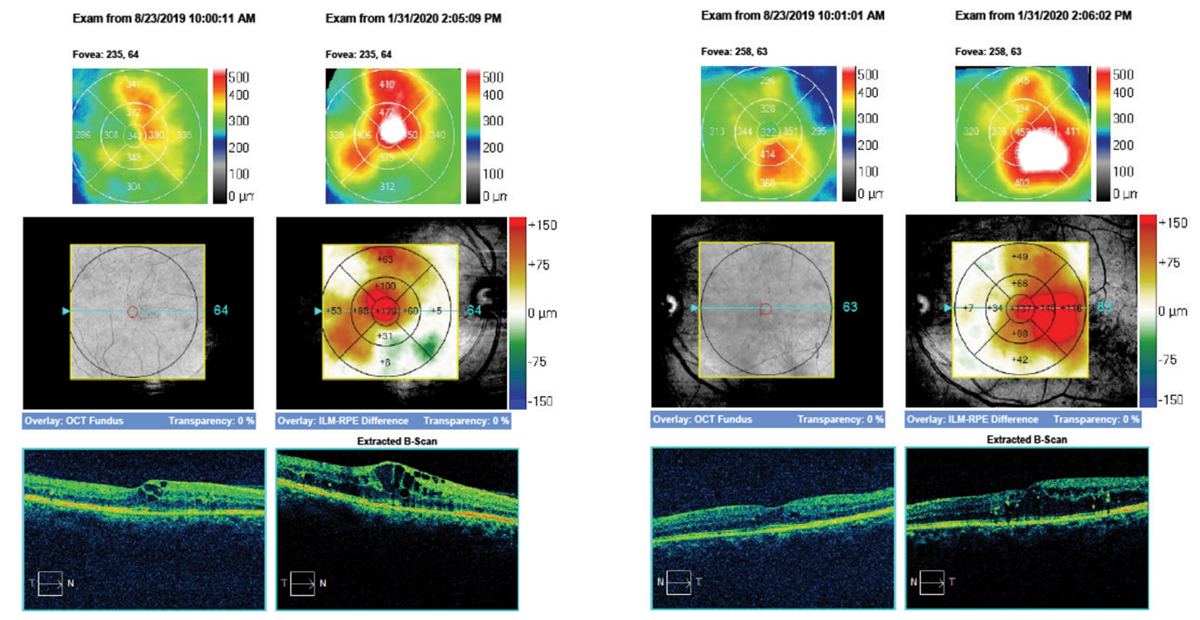 |
| Here, the same 55-year old patient’s macular cube change analysis of right and left eyes demonstrate the frank progression of DME over the course of six months. Click image to enlarge. |
Vitreoretinal surgery for rhegmatogenous retinal detachment. Statin therapy may have a potentially beneficial effect on vitreoretinal (VR) surgery outcomes. Statins exhibit anti-inflammatory, antioxidative, anti-fibroproliferative, and microvasculoprotective effects that contribute to photoreceptor survival, the retinal wound healing process and inflammation related to proliferative vitreoretinopathy (PVR) formation in eyes after surgery for rhegmatogenous retinal detachment (RRD).43,44 Research shows statins can reduce intravitreal levels of angiopoietin-2, vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2, which are factors associated with vascular permeability, inflammation, and fibroproliferation.45
According to a Finnish population-based cohort study, the use of simvastatin and atorvastatin was associated with a significantly lower risk of re-vitrectomy in patients whom previously underwent intervention for a RRD.46 Of note, rosuvastatin also lowered the repeat procedure rate but to a lesser degree than the aforementioned medications.46 Lipophilicity impacts on the pleiotropic effects of statins such as cell function, coagulation and inflammation. Although the pleiotropic effects of statins have been related to the outcomes of re-vitrectomy rates after RDD, additional randomized clinical trials are warranted to further determine the effects of statins on VR surgery outcomes.
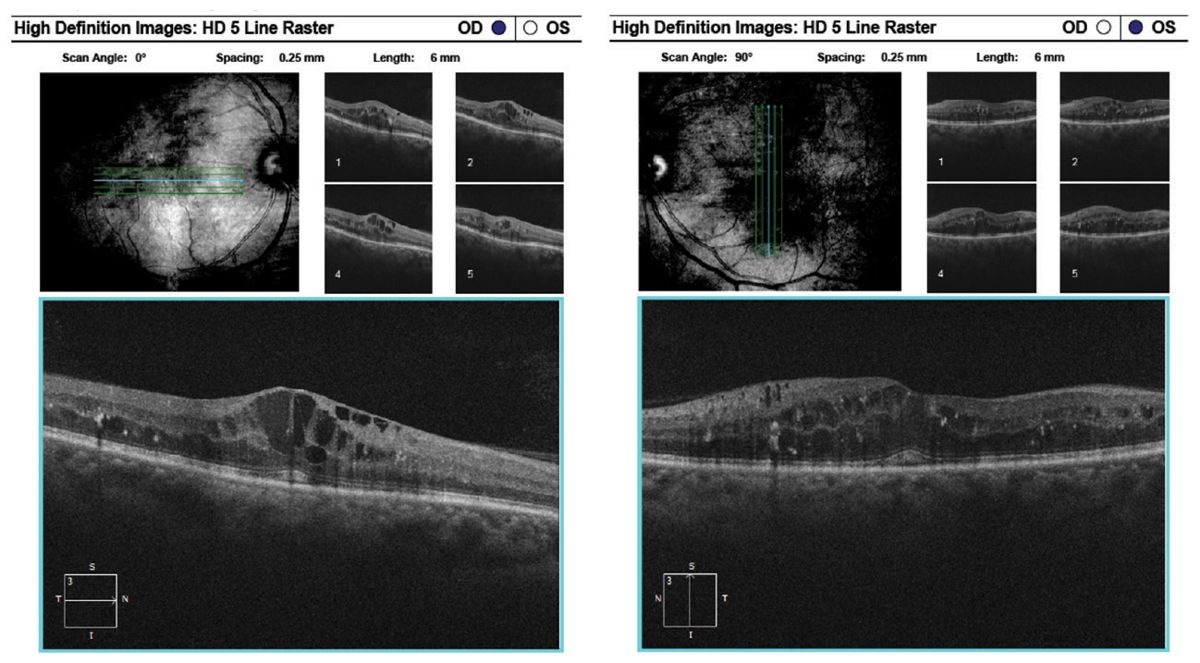 |
| Here, raster images of the previous patient show center-involved macular edema of the right eye and diffuse edema and retinal thickening of the left eye. Click image to enlarge. |
Age-related macular degeneration (AMD). Recent epidemiologic, genetic and pathological evidence shows AMD and atherosclerosis share several risk factors, leading to the hypothesis that statins may provide protective effects in AMD. There have been several reported mechanisms of how statins may aid in preventing AMD progression.47 Researchers believe the cholesterol-lowering, antioxidant, anti-inflammatory action, antiproliferative and anti-endothelial dysfunction effect of statins reduce the incidence and progression of AMD.48-51 Statins may also have the ability to preserve vascular supply to the outer retina and may inhibit secretion of MMP, which is involved in the development of choroidal neovascularization (CNV).47,52
Research on statins’ effect on AMD has yielded inconsistent and conflicting results. Some studies report a protective effect in early AMD, while others show an association between their use and AMD development at five years.53-61
The heterogeneity of AMD suggests that the effects of statins may vary by stage, reducing the development of drusen at the onset of AMD and having further anti-inflammatory effect on late-disease.58 Studies also suggest the need for a genetic analysis to understand whether the genotype can influence a response to statins as a therapeutic option.62 One factor impacting the conflicting results is the lack of standardization of statin dosages or individual lipophilicity for each class of drug.63
Choroidal neovascular membrane. Elevated intraocular levels of VEGF play an important role in the development of CNV and AMD. Research shows statins reduce plasma levels of VEGF and downregulate transcription factors involved in VEGF expression, thus potentially reducing the incidence and progression of CNV.51
Implications of statins on CNV development is not well understood with few relevant trials and observational studies.59 Interestingly, a large cross-sectional study of 3,090 patients with dry AMD found a larger proportion of statin users developed a CNV (29.3%) compared to non-statin users (23.3%); these results remained true after adjusting for age, sex, race, and comorbidity status.64
Retinal vein occlusion. Atherosclerosis, along with cardiovascular risk factors such as hyperlipidemia, diabetes and hypertension, contributes to the pathophysiology of retinal vein occlusion (RVO).65 Elevated cholesterol levels can change the plasma viscosity and alter platelet function, predisposing the blood to thrombosis and hemostasis.66 Research shows low-dose simvastatin promotes vascular repair through upregulation of VEGF and NO levels, while higher doses inhibited reparative processes and resulted in cell death due to depletion of intracellular cholesterol and disruption of key structures within the cells, which increases ischemia-induced neovascularization.67 However, an additional study found no preventive or therapeutic benefit of statins in high-risk patients.68 Very few studies have explored the role of statins in the management of RVO.
Despite the dearth of randomized trials focusing on statins and their effects on the eye, there is inconsistent evidence regarding their role in various eye conditions. At this time, the literature does not support the recommendation that eye care physicians change their practice patterns or recommend statins solely for improved ocular health. However, statins are medications ODs encounter every day. Optometrists need to be versed in the potential side effects and work to comanage the patent with their primary care doctor, cardiologist or internist. It’s prudent to advise patients to discuss their cholesterol levels with their primary care physician.
Drs. Zimbalist and Gentry practice at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Mo.
Dr. Ho practices at the Central Texas Veterans Health Care System Austin Outpatient Clinic in Austin, TX.
|

