Clinicians can think of the art and science of refraction on two levels: Simplistically, we are measuring the state of an eye’s focus and binocularity in order to attain best possible vision for our patients. But more than that, we are also directly and indirectly evaluating the quality of the ocular media, including cornea, aqueous humor, crystalline lens and vitreous humor. Even the condition of the retina can influence refractive results; for example, if there is edema or elevation from other causes. This article explores diagnosing and prescribing at the interface of refractive findings and ocular health by looking at some tough refractive cases commonly encountered in practice.
Prescribing Prism
As optometrists, we all know that refraction and binocular function are intimately related, and prescribing for patients who have a deviation of the visual axes is both challenging and rewarding. These patients present with a variety of asthenopic and diplopic symptoms. Extensive use of digital devices and sedentary occupations certainly haven’t helped. Given that patients cannot always regulate their visual tasks and device use— productivity demands and available technologies may limit patient comfort, in terms of ergonomics and duration of use—prescribing prism can have a huge impact on a patient’s well-being.
At the primary care level, clinicians must first rule out pathological processes prior to incorporating prismatic correction into the spectacle Rx. Although patients often expect the primary care eye doctor to provide treatment upon the initial visit, the possibility of pathologic origin requires a team approach. The most important step is to ensure that phorias and tropias are not due to pathology of the eye muscles or cranial nerves. This may involve consulting with the patient’s pediatrician, pediatric ophthalmologist, neurologist or neuro-ophthalmologist and blood testing and neuroimaging. Any new-onset binocular deviation that is noncomitant (i.e., variable according to gaze position) merits medical investigation. These consultations, along with a complete review of systems and an exam by the primary care OD, can help uncover potential systemic causes for extraocular muscle dysfunction.
| Fig. 1. Halberg clips are a great aid for measuring and demonstrating proposed refractive changes. |
There are many methods for measuring phorias and tropias, with von Graefe prisms and prism-bar neutralization being among the most common in our practice. Maddox Rod testing is helpful in quickly identifying a deviation as comitant vs. noncomitant, and prism can be incorporated to aid in quantitative measurement.
However, the method of measurement is not as critical as trial-framing the result. Practitioners can use Halberg clips to attach the proposed prism to the patient’s own spectacle frame (Figure 1). It may be helpful to ask the patient to read at near or to view a distance target, based on the nature of symptoms, for an appropriate period of time. While positive results of prismatic correction may be apparent immediately, allowing patients a little time with the trial Rx in place can be even more revealing. Using Halberg clips in-office may solve the issue for a limited timeframe, but if symptoms are variable or if they develop over several hours, Fresnel prism might be the better choice. Address the chief complaint at the specific working distance with prismatic trial.
Fusional reserves are also helpful in ascertaining the right degree of prism. For example, a patient who is symptomatic for near tasks may have unremarkable phorias, yet becomes diplopic when challenged by a minimal amount of base-out prism. If the eyes are unable to converge enough to overcome this base-out challenge, and the patient is symptomatic, a prescription of base-in prism for reading may be an effective and efficient remedy. Again, trial framing the prismatic Rx with a small amount of prism is helpful. Allow time for adaptation in the office, and present a near task similar to the one that generates the reading complaint. Having the patient test with a personal device such as a cell phone or tablet can be beneficial.
The degree and direction of visual axis deviation can help the primary care optometrist determine if prism is appropriate. We usually prescribe prism equal to one third to half of the binocular deviation. If the deviation is large, especially greater than 10 prism diopters, the prism can make the lenses unsightly with difficult adaptation. For example, a patient presenting with an 8 prism diopter exophoria may do well with an initial Rx of 1.5 prism diopters base-in for each eye. A larger prescription, in this case, may neutralize the deviation but present adaptation difficulties. A 20 diopter exodeviation would call for an unwearable amount of prism, if it had not been worn previously.
 |
| Fig. 2. Fresnel prism is an economical way to demonstrate prism without permanently fabricating it into the patient’s lenses. |
Patients who are unsuccessful trialing this prism should consult with a vision therapy specialist or eye muscle surgeon. Patients with binocular dysfunction may have difficulty with the most accurately prescribed prism, or find that the lenses are unacceptably thick or asymetrical in appearance. The key for the primary care optometrist is realizing when specialty care is needed. The true art of prescribing prism is shaping patient expectations, with a clear explanation of the diagnosis, treatment and expected outcomes. As with any other therapeutic procedure, the patient’s input is paramount in making the decision.
Other challenges the primary care optometrist might face include oblique prism and patients who have had multiple extraocular muscle surgeries without total success. The oblique prism tends to have variable results that may be best undertaken by a specialist. Binocular vision optometrists or pediatric ophthalmologists have experience prescribing prism of this nature. It is best to establish these experts in advance of the consultation. The multiple-surgery patient has a special set of needs, including muscle scarring and very poor fusional abilities. Clinicians may struggle with patients who do not appreciate induced diplopia on testing, as imbedded suppression would tend to indicate specialty care. A team approach of vision therapy—and possibly repeat surgery—is often indicated, in addition to a prismatic Rx.
Case Example
A 91-year-old pseudophakic white male with medication-controlled hypertension and diabetes presented with a four-year history of intermittent horizontal diplopia while driving. He carried a diagnosis of right sixth nerve paresis from another practice, when the diplopia occurred initially.
Neurologic work-up was negative for occult pathology. von Graefe testing revealed 8 prism diopters of esophoria at distance and orthophoria at near. Maddox Rod testing showed an eso deviation that increased on right gaze and decreased on left, confirming the original diagnosis.
Surgery and vision therapy were not practical options; thus, prismatic correction became a plausible choice. Patients tend to accept an initial prismatic correction equal to approximately half of the deviation measured. In this case, a trial of 2 diopters base-out in each eye produced a disorienting sensation that did not improve with office trial. The prism was reduced to 1 diopter base-out in each eye, resulting in better acceptance of the prescription and significant improvement of symptoms.
The patient had recently purchased new spectacles, creating a great opportunity to use Fresnel prisms (Figure 2). As a temporary plastic applique, we could test the efficacy of prism prior to having the spectacles remade. With the Fresnel prism in place, the patient’s acceptance at follow-up was fine, and he reported significant reduction of diplopic symptoms while driving. We issued a new spectacle Rx incorporating the prism. While Fresnel prisms are great for trial and demonstration, the polymer applique and adding surfaces through which the patient sees can reduce objective and subjective acuity.
Prescribing Spectacles for the Rigid Gas Permeable Wearer
Patients who wear rigid gas permeables (RGP) and want spectacles represent one of our most challenging refraction scenarios. Even the perfectly-fit RGP can alter the corneal shape, thus altering refractive error. Simplistically, the spherical base curve of the RGP cannot match the complexity of the aspheric cornea, and compression of the corneal epithelium is inevitable. In the days after the lens is removed, the effect of this compression gradually reduces, creating a variable refractive error. Add in any issues with corneal edema and inflammation, and the refraction becomes a moving target. At least one line of reduction in best-corrected acuity is not unusual.1
In my practice we measure the spectacle prescription about 20 minutes after the RGP is removed, which may be done in concert with routine pupillary dilation. While this does not account for all variability produced when a lens is removed, it accommodates the patient’s need when removing lenses. That is, the patient wants to wear the spectacles in real time—upon removing the lenses. The precorneal tear film has somewhat stabilized during the 20 minute wait, and any wetting solution and mucin has blinked away. Often, prior generations of clinicians asked patients to remove lenses three days before the exam to allow the cornea to return to its “normal” shape. However, patients want to wear spectacles immediately, not three days after removing the lenses.
The key when prescribing spectacles for the RGP patient is to consider the sphere and cylinder powers and axes of the prior Rx. A simple prescription of the current subjective refraction is a formula for a spectacle remake. A diopter change in subjective refraction might result in an Rx of half that amount. Similarly, cylindrical adjustments should be a fraction of the change measured. RGP patient counseling on the variability of spectacle vision is another key to success and should be emphasized at each visit.
Practice Pearls
|
Case Example
A 60-year-old white female has a long history of contact lens wear, having switched from PMMA lenses to RGPs when they became available in the ’80s. She corrects to 20/20 in each eye with her contact lenses. Her chief complaint is that she cannot see well with last year’s spectacles. The lenses themselves fit in a superior, lid-controlled fashion, with base curves 0.75 diopter flatter than the flat central keratometric reading. The patient’s prior eye care practitioner performed spectacle refraction after the lenses were removed for a three-day period. Her habitual spectacles were -3.75-1.00x180, 20/40 OD and -4.00-1.25x180, 20/30 OS.
Our spectacle refraction, performed 20 minutes after lens removal, produced OD -3.00-1.00x180, 20/30 OD and -3.50-0.75x180, 20/25 OS. The reduced minus seems to be associated with the timing of the refraction on last year’s exam. Contact lenses fitted “flat” centrally tend to have an orthokeratologic effect that begins to unwind itself during the three days that the prior doctor had indicated for a rest from the lenses. The cornea begins to return to its more natural, steeper shape, thus affecting the spectacle refraction.
Practice Pearls
|
Figure 3 shows the patient’s corneal topography immediately post-removal of the contact lenses. At a glance, the graphics would seem to indicate keratoconus, with inferior steepening and irregularity of the cornea. However, the statistical indices reveal no suspicion of keratoconus, and the molded corneal shape corresponds to the high-riding contact lens position.
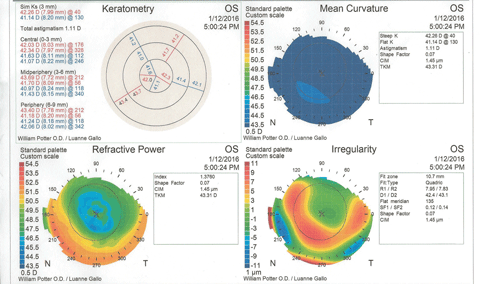 |
| Fig. 3. Post-removal corneal topography shows distortion and some inferior corneal steepening that could be misinterpreted as keratoconus. The eccentricity value does not support a keratoconus diagnosis. Click image to enlarge. |
Prescribing Spectacles for the Diabetic Patient
Patients with diabetes often present challenges when prescribing spectacles as well. Elevated blood sugar can produce changes in the crystalline lens, altering clarity, refractive index and curvature. Blur from diabetic pathology itself can be a confounding factor. Visual variability can be due to optic neuropathy, which can be frank or subtle, and may not be apparent on clinical exam. Patients with diabetes may also be susceptible to dry eye syndrome, causing further difficulty with visual acuity and function.2,3 Diabetic retinopathy—with its hemorrhages, microaneurisms and neovascularization—tends to have little influence on refractive results unless there is significant macular edema, which can cause a hyperopic shift due to its shortening of axial length.
Practice Pearls
|
There is much debate regarding refractive change in these patients. Is there a myopic shift, or hyperopic? Literature evidence is sparse, though discussion with colleagues and some texts suggest a consensus for myopic shift.4 The classic Borish text, Clinical Refraction, refers to osmotic dehydration of the crystalline lens as a cause for these changes.5 There is also the possibility of a temporary hyperopic shift as control is attained.6 Researchers who evaluated risk factors and the direction of refractive change in diabetic patients found that, over time, type 1 diabetics were likely to be more myopic than those with type 2 diabetes.7 However, a longer duration of type 1, and the presence of proliferative retinopathy, indicated more strongly for hyperopic shifts.7 Acutely, refractive shifts tend to be in the myopic direction, although hyperopic shifts can occur during aggressive attempts at controlling blood sugar.7
Our practice has experienced a number of large hyperopic shifts on initial presentation, with some support in the literature.8 As the natural history of the patient’s blood glucose control is usually not specific, many may have been seen when their control was improving. Again, the poorly understood pathophysiology of the diabetic refractive shift leaves these events subject to speculation.
The typical diabetes patient with refractive changes will present with a chief complaint of a profound visual change in each eye. The sudden onset and the relatively extreme dioptric change can help differentiate diabetic involvement from physiologic changes that naturally occur in myopes and hyperopes. A cursory refraction will typically reveal two or more diopters of variability in sphere power compared with the patient’s habitual Rx. In our experience in a large OD/MD practice, patients whose fasting blood sugars regularly touch the 200 range are prime candidates for sudden refractive shift.
The challenge is meeting the patient’s visual needs following initial diabetic treatment. Stability may take six weeks to attain, and patients may have driving or reading needs.5 We prescribe a “temporary” installation of lenses. Patient education is key to ensure they understand the Rx will change, and family practitioners usually help reinforce the notion of variable vision. Keep the features of the temporary lens simple, usually without progressive, tint or antireflection features. In many cases, a pre-fabricated, OTC spectacle can be specified as a temporary Rx. Schedule a follow up in six weeks to check for the resolution of visual findings. Patients occasionally return and indicate that vision has long since returned to the baseline. In the meantime, we feel that our strongest obligation is to provide for the patient’s visual needs to drive safely, both in terms of comfort level and being able to pass a state motor vehicle department vision exam.
Case Example
A 48-year-old white male first-time patient presented with the unusual complaint that he “could suddenly read without his reading glasses, and street signs were blurry.” The patient reports hydrochlorothiazide for systemic hypertension as his only medication. He had a significant history of type 2 diabetes mellitus in the family. His uncorrected acuity was 20/80 OD and 20/80 OS, with manifest refraction of -2.00 sphere in each eye, to 20/20. Unaided near acuity was 20/20 in each eye, and was not helped by his habitual reading Rx of +1.75 spheres in each eye.
During the examination, we contacted the patient’s prior eye care provider, who revealed that last year’s examination showed a plano sphere Rx in each eye for distance, with a +1.75 add for reading, and a normal dilated examination with no cataract or other health issues. Similarly, our current exam was essentially normal and revealed no cataract and no diabetic retinopathy or optic neuropathy.
Clearly, a sudden two diopter refractive shift is cause for concern. While incipient nuclear sclerosis cataracts can cause significant myopic changes, this patient’s crystalline lenses were perfectly clear. We sent the patient off to his primary care physician (PCP), requesting that she rule out diabetes mellitus. The patient’s laboratory testing revealed a fasting blood sugar of 210 and a hemoglobin A1C of 9.7. His PCP initiated diabetic therapy in the form of dietary education, exercise regimen and oral Metformin. The patient’s laboratory values returned to the more acceptable fasting blood sugar of 110, with A1C of 6.9, in the ensuing six-week period.
This patient was well-served by temporary use of OTC readers, recommended at +1.75. As there was no cataract formation or retinal pathology, vision returned to baseline after six weeks of treatment.
Dr. Potter is chief of optometry and contact lens services at Millennium Eye Care in West Freehold, NJ, a multi-subspecialty optometry/ophthalmology practice.
|
1. Ruiz-Montenegro J, Mafra CH, Wilson SE, et al. Corneal topographic alterations in normal contact lens wearers. Ophthalmology. 1993 Jan;100(1):128-34. 2. Milton H. Diabetes and Dry Eye: The Forgotten Connection. Review of Optometry. 2010 Sept;147(9). 94-105. 3. Tong L, Waduthantri S, Wong TY, et al. Impact of symptomatic dry eye on vision-related daily activities: The Singapore Malay Eye Study. Eye. 2010;24:1486-91. 4. Milder B, Rubin M. The Fine Art of Prescribing Glasses Without Making a Spectacle of Youself. 3rd ed. Gainsville, FL: Triad Scientific Publishers;2004:96. 5. Borish I. Clincal Refraction. 3rd ed. Chicago, IL: The Professional Press; 1975:1048-9. 6. Sonmez B, Bozkurt B, Atmaca A, et al. Effects of glycemic control on refractive changes in diabetic patients with hyperglycemia. Cornea. 2005 July;24(5):531-7. 7. Klein B, Lee KE, Klein R, et al. Refraction in adults with diabetes. Arch Ophthalmol. 2011 Jan;129(1):56-62. 8. Tai MC, Lin SY, Chen JT, et al. Sweet hyperopia: refractive changes in acute hyperglycemia. Eur J Ophthalmol. 2006 Sept-Oct;16(5):663-3. |

