|
|
 |
History
A 37-year-old female presented to the office with a chief complaint of blurry vision at near. Her systemic history was remarkable for arrhythmia and hypertension for which she was properly medicated. She had no known allergies.
Her last eye exam was estimated at being seven years ago with no reported abnormalities or unusual diagnoses.
Diagnostic Data
The patient’s best-corrected acuity was 20/20 OU at distance and 20/25 OU at near. Her external examination was normal and there was no afferent pupillary defect.
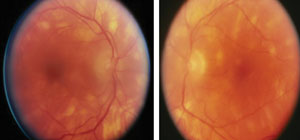
Refraction uncovered mild hyperopia measuring 0.50 DS. The cornea and internal ocular health examination was normal, OU. Goldmann intraocular pressures measured 14mm Hg, both eyes. The pertinent dilated fundus findings are demonstrated in the photograph.
Your Diagnosis
How would you approach this case? Does this case require any additional tests? What is your diagnosis? How would you manage this patient? What’s the likely prognosis?
Discussion
Important questioning might have included any complications with night vision or going from a light to dark or dark to light environment. Optical coherence tomography and photodocumantation are beneficial in these cases. Electrodiagnostic testing (full field and multifocal electroretinogram [ERG] is also helpful. Fluorescein angiography might be completed by retinology to rule the presence of choroidal neovascularization or vascular leakage.
|
|
|
|
|
Fundus photographs of a 37-year-old female with a chief complaint of blurry vision at near. What follow up questions might you consider based on these findings? |
The diagnosis in this issue is Birdshot retinochoroidopathy (BRC). BRC is sometimes referred to as vitiliginous chorioretinitis and is a rare ocular disorder that was named and delineated as a separate clinical entity by Ryan and Maumenee in 1980.1,2 Known to be a bilateral, progressive, retinal-choroidal vascular-inflammatory disease with a strong association to middle-aged individuals of Caucasian Northern European decent, birdshot retinochoroiditis produces a diffuse posterior choroidopathy with an associated anterior segment reaction, vitritis and retinal vasculitis.1-9 The typical age of presentation is documented as the 4th-5th decades of life with a slight female predominance.6 As the disease progresses, profuse retinal-vascular leakage ensues with resultant retinal, macular and disk edema developing.1-9 The fundus lesions are often described as “creamy,” small (less than 1 disc diameter) and scattered throughout the entire fundus.1-8 As the retina and disc swell and cystic macular edema (CME) develops, visual acuity is variably affected.1-5 Individuals presenting with a history of the ailment for a duration longer than 30 months have a higher incidence of visual acuities 20/50 or worse.8 Visual loss can progress to levels as poor as 20/200.10
In these cases the fundus exhibits a characteristic patterned distribution of depigmented spots with an absence of the classic, adjacent hyperpigmentation reaction.2 Traditionally there are no pathologic alterations about the optic disc, even when the optic disc itself succumbs to the effects of process.4 Early complications of the disease include a variable inability to see in the dark, visual field deficits, epiretinal membranes (with the potential for macular hole development), venous sheathing, retinal neovascularization with or without recurrent vitreous hemorrhage, subretinal neovascular membranes occurring both in the juxtapapillary and perimacular regions along with the end stage complication of optic atrophy and changes that mimic retinitis pigmentosa despite the classic reputation of an absence of this type of change.1-9
The diagnosis is made classically upon its appearance and ocular findings.5 The fluorescein angiography appearance demonstrates mild hyperfluorescent patches which correlate to the areas of hypopigmentation. There is often mild vascular leakage, late optic disc staining and the macular pettaloid appearance classic of CME.5
The sensory retina is defined by its cellular elements; the retinal pigment epithelium, a photoreceptor and two neurons (horizontal, bipolar, amicrine cells and ganglion cells). Those cell types which support the neurosensory retina, lying posterior to the retinal pigment epithelium: Bruch’s membrane and the choroid, are collectively recognized as the subretinal strata. Diseases which affect the Bruch’s membrane and choroid are referred to as subretinal diseases. BRC is considered a choroidopathy and hence a subretinal disease.
The specific etiology of birdshot choroiditis remains unknown.1-15 The majority of patients possess autoimmune human leukocyte antigen disease (HLA), where the body fails to recognize self from nonself.1-5 The HLA A29 marker is its specific identifier.1,4,5 Lymphocyte reactivity to the retinal S antigen also points to an autoimmune etiology.4 The distribution and appearance of the lesions map them to the major choroidal veins.4 The preponderance of evidence suggests that this is a single, unique disease rather than a conglomeration of concomitantly occurring disorders because in each case there is core similarity of ophthalmologic appearance, clinical course and association with the HLA-A29 marker.4
Under the influence of released inflammatory cytikines, retinal blood vessels (capillaries and retinal arterioles) become more permeable creating an environment conducive to the formation of cystoid macular edema.1-5 CME is the predominant cause of lost visual function.1-14 Defects in the visual field can be correlated to the areas of choroidal depigmentation.1-16
Treatment for BRC is not always required. In some instances, where there is no threat to macular integrity and no loss of function, careful photodocumentation and monitoring will suffice. When vision is threatened or macular edema ensues, intervention should be considered. Periocular and systemic steroidal preparations are the mainstay of treating the disease.5,9,11,12 Here the goal is to slow or arrest the inflammatory process, decreasing vascular leakage and preserve functioning by limiting impingement on macular structures.5-14
Treatment
Azathioprine is a commonly used immunosuppressive agent designed to be deployed in cases where inflammation is unsuccessfully controlled with oral or intravenous steroids.11
Given the inflammatory-vasculopathic nature of birdshot retinochoroidopathy the agent is suitable for augmenting disease control along side other preparations. It also induces an environment conducive to less steroidal medication.11
The principal indications for azathioprine with respect to birdshot choroidopathy is uncontrolled disease while approaching maximum systemic steroid therapy.5,11,12
The medication, typically prescribed a year or more demonstrates an affinity for decreasing the relapse rate and for reducing the total steroid dosage.11
Variable improvement of visual acuity or at least maintenance of acuity is the rule more than it is the exception.11
Other medications in the same class, possessing a similar mechanism of action are methotrexate and cyclosporine.5,11-14
Interestingly, researchers have uncovered a link between cyclosporine reduction and adjunctive ketoconazole usage.12
The regimen appears to be safe and efficacious.12
The combination of systemic corticosteroids and immunomodulating agents is sometimes referred to in the literature as corticosteroid-sparing systemic immunomodulatory therapy (IMT).13
Without question this particular strategy has gained momentum as the current standard with prompt induction clearly able to preserve tissue structure, architecture and function.12-14
Since some patients may be apprehensive with regard to the effects of an intricate medicinal cocktail, another viable option is intraocular corticosteroid injection.15 Intravitreal triamcinolone injection has demonstrated reasonable efficacy as a therapy for BRC.15 Its advantages include a swift, stabilizing effect while abating the potential for systemic side effects often produced by the intravenous and oral preparations.13 Of course, the inherent complications of this class of medicine remain and include premature lens changes and increased intraocular pressure.15
Intravenous polyclonal immunoglobulin (IVIg) has been successfully used in a number of autoimmune conditions.16 Investigators have used this modality as an alternative to traditional therapy with some success.16
While laser photocoagulation for the exuding lesions and the uveitic-based CME is not specifically recommended, even in advancing cases, oral acetozolamide therapy can be attempted to reverse the progression of macular leakage.17 As a last resort, when all other standard and current modalities have failed to detain runaway macular swelling, pars plana vitrectomy (PPV) with intravitreal triamcinolone application has recently shown promise as a procedure, which at the least, may temporarily stop the process.17 Unfortunately, frequent complications include premature cataract formation and ocular hypertension.17
Monitoring
Fluorescein angiography, indocyanine videoangiography and other instruments which measure or image retinal thickness and retinal structures along with electrodiagnostic equipment can be used to help monitor the disease and its rate of progression.5,18
Measurements can be taken before and after treatment to improve the accuracy of this approach.5,18
The long-term prognosis of the disease is guarded.
End stage disease can result in sensory retinal and optic nerve atrophy with resultant, permanent visual consequences.
The amount of visual loss is typically proportional to the chronicity and extent of the cystoid macular edema.
Aggressive systemic corticosteroid therapy is not without its own set of potential side effects including premature cataract formation, cystoid macular edema, steroid induced-glaucoma, epiretinal membrane and retinal detachment.
1. Fich M, Rosenberg T. Birdshot retinochoroidopathy in monozygotic twins. Acta Ophthalmol (Copenh). 1992;70(5):693-7.2. Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;89(1): 31-45.
3. Kaplan HJ, Aaberg TM. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980 ;90(6):773-82.
4. Priem HA, Oosterhuis JA. Birdshot chorioretinopathy: clinical characteristics and evolution. Br J Ophthalmol. 1988;72(9):646-59.
5. Moorthy RS, Jampol LM. Posterior uveitis of unknown cause. In: Yanoff M, Duker JS. Ophthalmology 2 nd ed. Philadelphia, Mosby 2004:1219-28.
6. Shah KH, Levinson RD, Yu F et al. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50(6):519-41.
7. Willermain F, Greiner K, Forrester JV. Atypical end-stage birdshot retinochoroidopathy. Ocul Immunol Inflamm. 2003;11(4):305-7.
8. Herbort CP, Probst K, Cimino L et al. Differential inflammatory involvement in retina and choroïd in birdshot chorioretinopathy. Klin Monatsbl Augenheilkd. 2004;221(5):351-6.
9. Nenciu A, Stefan C. Birdshot retinochoroidopathy. Oftalmologia. 2003;58(3):13-20.
10. Thorne JE, Jabs DA, Peters GB et al. Birdshot retinochoroidopathy: ocular complications and visual impairment. Am J Ophthalmol. 2005;140(1):45-51.
11. Greenwood AJ, Stanford MR, Graham EM. The role of azathioprine in the management of retinal vasculitis. Eye. 1998;12(Pt5):783-8.
12. Silverstein BE, Wong IG. Reduction of cyclosporine dosage with ketoconazole in a patient with birdshot retinochoroidopathy. Am J Ophthalmol. 1998;125(1):106-8.
13. Kiss S, Ahmed M, Letko E et al. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005;112(6):1066-71.
14. Becker MD, Wertheim MS, Smith JR et al. Long-term follow-up of patients with birdshot retinochoroidopathy treated with systemic immunosuppression. Ocul Immunol Inflamm. 2005;13(4):289-93.
15. Shah A, Branley M. Use of intravitreal triamcinolone in the management of birdshot retinochoroidopathy associated with cystoid macular oedema: a case study over a three-year period. Clin Experiment Ophthalmol. 2005;33(4):442-4.
16. LeHoang P, Cassoux N, George F et al. Intravenous immunoglobulin (IVIg) for the treatment of birdshot retinochoroidopathy. Ocul Immunol Inflamm. 2000;8(1):49-57.
17. Gutfleisch M, Spital G, Mingels A et al. Pars plana vitrectomy with intravitreal triamcinolone: effect on uveitic cystoid macular oedema and treatment limitations. Br J Ophthalmol. 2007;91(3):345-8.
18. De Geronimo F, Glacet-Bernard A, Coscas G et al. Birdshot retinochoroidopathy: measurement of the posterior fundus spots and macular edema using a retinal thickness analyzer, before and after treatment. Eur J Ophthalmol. 2000;10(4):338-40.
19. Comander J, Loewenstein J, Sobrin L Diagnostic testing and disease monitoring in birdshot chorioretinopathy. Semin Ophthalmol. 2011;26(4-5):329-36.

