 |
A 30-year-old female patient presented complaining of progressively worsening red eyes over the past three days. She had no frank pain, but felt that her eyes were generally uncomfortable and “unwell.” She had a fair amount of serous discharge and reported that her eyelids were matted shut with dry, crusty tears when she arose. She had no itching or photophobia. She acknowledged a mild upper respiratory infection (URI) a little more than a week ago.
Her vision was minimally reduced at 20/25 in each eye. There was no mucopurulence, but her eyes were watery with serous discharge. She had a grade 2 diffuse conjunctival injection. There were several faint subepithelial infiltrates (SEI) in both corneas and perhaps one or two white blood cells in the anterior chambers. Inspection of the palperbral conjunctiva revealed a pronounced follicular response in each eye. Palpation of her preauricular nodes showed the glands to be swollen and tender bilaterally. She was concerned that she had “pink eye.”
We explained that she actually had a form of viral conjunctivitis, most likely epidemic keratoconjunctivitis (EKC). After a detailed discussion of the infectious nature of the condition, we recommended copious artificial tears and cold compresses and prescribed a mild topical steroid to give her symptomatic relief.
For many years, when encountering any form of suspected viral conjunctivitis, we could do little other than recommend palliative measures to increase comfort. There was no standard therapy, and practitioners employed cold compresses, nonsteroidal or steroidal medications and artificial tears in varying combinations dictated by personal algorithms, due to the fact that the offending viruses really weren’t susceptible to any known treatment.
However, that may be changing with a new compound currently under investigation.
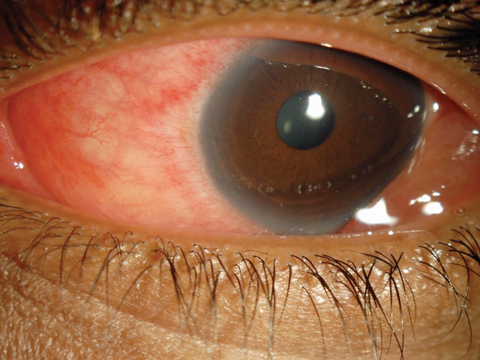 |
| For EKC (not “pink eye”) patients, such as this 30-year-old woman, a new option for relief may soon be available. Click image to enlarge. |
Monitoring
The two frequently encountered forms of viral conjunctivitis are pharyngoconjunctival fever (PCF) and EKC.1-11 Both forms are difficult to distinguish from one another clinically. PCF is marked by fever, sore throat, history of URI and follicular conjunctivitis.1,2,4,7,9-11 The condition may be unilateral or bilateral, but classically presents in one eye and subsequently spreads to the other.1-8 The cornea is rarely affected, and infiltrates are uncommon.5 Preauricular lymph nodes may be palpable and tender. The virus has a self-limiting infectious period of 14 to 30 days.7,8 The condition is contagious through the entire clinical period. Principal ocular symptoms of PCF include diffuse conjunctival redness, watery discharge and epiphora.
EKC presents as a unilateral or bilateral inferior palpebral follicular conjunctivitis with epithelial and subepithelial keratitis.12,13 SEI do not occur in every instance. When observed, they are typically concentrated in the central cornea.12-15 These localized gatherings of leukocytes can persist for months or longer. It is speculated that using topical steroids may prolong the infiltrative response. This condition is also highly contagious.9-12 Viral conjunctival infections are thought to be spawned by viral molecules transmitted either by airborne respiratory droplets or direct transfer from fingers to the conjunctival surface.1-4,7-16
Differentiating the various causes of conjunctivitis can be daunting, often leading to a “shotgun approach” in an attempt to treat everything that may be present, including bacteria. The point-of-care diagnostic service AdenoPlus (Quidel) uses technology based on lateral flow immunochromatography to identify adenoviral antigens.17,18 Once clinicians obtain the sample, a result is available within 10 minutes and can minimize misdiagnosis.
Therapies
Viral conjunctivitis is contagious but self-limiting. Management currently involves increasing patient comfort and patient education so as to limit spread of the condition. Patients should stay home from work or school until the discharge has ceased. Medical management may range from supportive cold compress and tears to topical vasoconstrictors, topical nonsteroidal anti-inflammatory agents and topical steroids BID to QID. Many practitioners will avoid steroids based on the fact that they suppress the immune system; while these medications may help to improve overall comfort, they also prolong clearance of the virus.
Recently, practitioners have employed a Betadine (povidone-iodine, Purdue) rinse of the ocular surface for viral conjunctivitis, despite the fact that this is an off-label use. The eye is topically anesthetized, and several drops of diluted povidone-iodine are placed into the palpebral fissure, bathing the conjunctivae for 10 to 30 seconds, and then rinsed away. Unfortunately, no standards exist for dilution or specific indications for use of this agent. Patients are often quite uncomfortable once the anesthesia wears off. Most importantly, success is anecdotal and few studies assess if this was even helpful.19
A better method may be on the way in the form of a fixed-combinaton antimicrobial/ anti-inflammatory medication. A double-masked study comparing 0.1% dexamethasone/0.6% povidone-iodine (SHP640, Shire) against povidone-iodine (PVP-I) and vehicle in 144 patients with adenoviral conjunctivitis has recently been completed. To be included in the study, patients must have had a positive AdenoPlus test, reported signs and symptoms of adenoviral conjunctivitis of at least five days, had a diagnosis of suspected acute adenoviral conjunctivitis in at least one eye, plus watery discharge and bulbar conjunctival redness.
Patients received one drop in both eyes four times a day for five days. At day six, the percentage of patients with clinical resolution (absence of watery conjunctival discharge and bulbar conjunctival redness) was 31.3% for the SHP640 group, 10.9% in the vehicle group and 18% in the PVP-I group. Adenoviral eradication was significantly higher in the SHP640 group (79.2%) compared with vehicle (56.5%) and numerically higher than the PVP-I group (62.0%). Adenoviral eradication was noted in both non-vehicle groups as early as day three, as reported at the ARVO meeting in 2017.20 Additional Phase III studies are currently underway for the treatment of both viral and bacterial conjunctivitis.
Should ongoing clinical trials prove this combination medication to be efficacious and safe, we may soon have a therapy that actually eradicates viral particles and hastens disease resolution, allowing us to go beyond simple palliative therapy. Should antibacterial efficacy also be shown, one medication can possibly be used for cases where we are clinically uncertain whether the origin is viral or bacterial.
1. Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310(16):1721-9. |

