Providing care for your patients during their recovery from cataract surgery can be exciting and gratifying. Few experiences will cement patients to your practice like regaining their vision; it will also help your clinic operate at the peak of its capacity. Most patients have a straightforward recovery, and only a few require more attention. If any serious problems present, your surgeon is standing by, ready to assist.1
Each month, our clinic and the community optometrists we serve see hundreds of cataract patients through their healing process. This article describes the sequence and elements of an uncomplicated recovery from cataract surgery and then discusses how to handle some of the more common complications.
| This patient—who had an IOP of 4mm Hg—displays corneal waffling. We saw no active leak, but the patient had gently rubbed the eye an hour before his one-day exam. |
The Uncomplicated Course
The vast majority of cataract cases undergo an uncomplicated and predictable path; in the United States today, more than 97% of all cataract cases unfold successfully.2 Timeline, medications and care have all been standardized for decades.
Medications. All cataract patients will require medications postoperatively to protect them from infection, inflammation and pain, but a wide variation exists in the specific medications and dosages used by individual surgeons. All formulas include an antibiotic to protect against endophthalmitis and a steroid to control inflammation. A steroid used for less than a month can be stopped abruptly when the bottle is empty, although many clinics will ask for the more traditional taper.
Some clinics use a nonsteroidal anti-inflammatory drug (NSAID) to complement the steroid in controlling inflammation and pain, while others do well without them. Regardless, NSAIDs are frequently prescribed for patients whose eyes have a high risk of developing cystoid macular edema (CME) or inflammation: this includes cases of diabetic retinopathy, epiretinal membranes, a history of retinal vein occlusion or macular degeneration.
A growing movement urges doctors to skip some, or all, post-op drops in favor of an injection usually containing a steroid and an antibiotic. In these cases you must be familiar with your surgeon’s mixture and its expected performance. This approach can produce some harmless but unusual visual effects immediately after surgery and has a rare but significant risk of a dangerous reaction to the medication.3
The one-day exam. Use the first postoperative exam to ensure that the surgery was carried out well, to verify that the patient understands their responsibilities and to answer their immediate post-op questions and concerns. The one-day exam must include a history, measurement of visual acuity (VA), an auto-refractor reading or pinhole acuity, an intraocular pressure (IOP) check and a slit-lamp exam.
For most patients, normal symptoms at the one-day exam include blur, foreign body sensation, ache and redness. Normal findings include reduced VA (typically around 20/25 to 20/60), a small ptosis (from the spring clamps used during surgery), residual dilation, mildly elevated IOP, injection and cells and flare in the anterior chamber.
There will be a primary incision, either in the temporal cornea or in the superior conjunctiva, along with one or two small corneal port incisions. Subconjunctival hemorrhages are common, especially following femtosecond-laser assisted surgery and among patients taking anticoagulants. Often, you will see some mild keratitis; grade 2+ or less should only need the diligent application of artificial tears to restore comfort. There will usually be some disruption of the endothelium (“snail tracks”) and small fragments of capsular debris in the anterior chamber; these are inconsequential and self-resolving. The intraocular lenses (IOL) should be well-positioned. Often, patients will feel sore, have a mild headache or will have slept badly; it is appropriate for them to resort to their over-the-counter oral NSAID of choice for this. The retina need not be examined at the one-day exam unless you or the surgeon have specific concerns.
Any comanaging optometrist must be comfortable grading anterior chamber inflammation, as this is used to judge progress throughout the post-op period (Table 1).
It is important at this time to confirm that the patient understands their drop regimen. Exhorting frequent artificial tear usage and the vigorous shaking of any steroid suspensions can avoid many subsequent panicked late-night phone calls. It’s also a good time to remind patients of their post-op restrictions, which usually include avoiding eye-rubbing, make-up or tap water near their eyes.
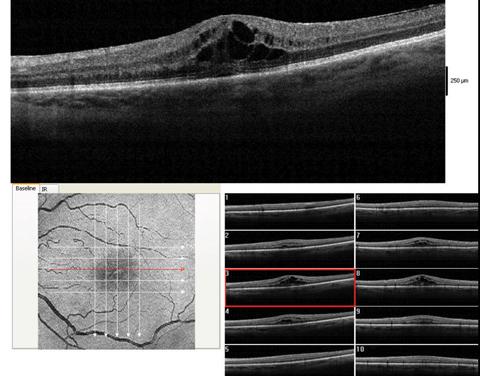 |
| These images show a moderate macular edema, diagnosed four weeks after surgery. With 1% prednisolone acetate QID and flurbiprofen QID, this patient recovered to 20/25 over the course of two months. Click image to enlarge. |
The one-week exam. Every patient should be seen between seven and 14 days after each eye’s surgery; this exam is used to verify that the incisions have healed enough to discontinue the patient’s antibiotics, monitor their refractive state and, often, to check for a satisfactory outcome so as to green-light their fellow eye for surgery. If so prescribed, you will ask the patient to begin tapering their steroid. The early signs of endophthalmitis can occur at this point, as well as CME, so be wary of unexplained inflammation, pain or poor vision.4
Although mild anterior chamber inflammation and mildly reduced vision are standard, all findings should be stable or improved compared with the one-day exam. The one-week exam must include a history, a measurement of aided and unaided VAs, an IOP check and a slit-lamp exam of the anterior segment. A dilated fundus exam is called for only if there are concerns.
The one-month exam. Typically, the patient will have one final exam three to six weeks after surgery. This one must include a dilated fundus exam to confirm the patient is well-healed and stable, and also a final postoperative refraction and prescription. The patient should be relatively asymptomatic, with the exception of refractive error complaints.
If all has gone well, this exam transitions the patient back to their regular eye care schedule. Be sure to review their ocular health, be explicit about the recommended frequency for eye exams and discuss the importance of adequate ultraviolet light protection now that their cataracts are gone. You may ask them to stop their drops at this point.
Expectations. If a patient enters into surgery expecting an improvement in vision after a measured recovery, a significant amount of work instilling eye drops, absolute presbyopia and some amount of residual refractive error, that patient will likely be pleased at every step. If you have the misfortune of caring for a patient who is expecting a perfect outcome, you will have to spend some time working through their unrealistic expectations. Any experienced surgery center will work hard to ensure that all patients have an accurate understanding of what the cataract surgery process will and will not deliver.
Premium IOLs. These introduce a different set of expectations for the postoperative period. These patients have invested in an IOL expecting higher visual performance and will require more care.
Toric IOLs can greatly reduce the patient’s astigmatism; use each postoperative exam to verify the patient’s refraction and satisfaction. The toric IOLs now available in the United States can correct corneal astigmatism between 1.03D and 4.11D—they don’t eliminate irregular astigmatism or astigmatism higher than 4.11D.
Multifocal IOLs are expensive and high performing, but require a significant amount of education and careful screening of candidates. Patients tend to have high expectations and some anxiety about their vision, and you will need to be well-versed in the details of the multifocal IOLs preferred by your surgeon to adequately counsel your patients. Appropriate expectations are crucial, and you must carefully counsel patients preoperatively to expect glasses wear for some specific tasks after surgery. Visual performance can increase for up to six months after implantation of multifocal IOLs as the patient’s neural pathways grow increasingly facile at working with an altogether new way of seeing, and your encouragement can help tremendously along the way.
|
Table 1. Grading Anterior Chamber Cell
|
Complications
While most cataract patients recover without a hitch, a few may encounter one of these complications:
IOP rise. We often see pressure spikes, whether from retained viscoelastic, or from the impact of inflammation on the trabecular meshwork, after surgery. Fortunately, this spike is transient and usually resolves within the first few days.5
If the optic nerve is healthy, our clinic will only treat the IOP if it is greater than 30mm Hg by Goldmann tonometry. If the pressure is 30mm Hg to 35mm Hg, often a single drop of brimonidine suffices; if it is higher than 35mm Hg, we will instill brimonidine and sometimes timolol and re-measure the IOP every 30 minutes until the pressure sinks below 30mm Hg. Prostaglandins are next to useless in this case due to their slow action. If the pressure is slow to recover we prescribe a bottle of brimonidine BID for a week. In general, we avoid “burping the wound” due to the risk of infection from backflow.
If the patient has glaucoma, IOP will require more aggressive treatment, based on the glaucoma severity and the stubbornness of the pressure.6 We keep a bottle of acetazolamide 250mg tablets in the clinic for use in dangerous situations; we give them at a QID dosage until IOP returns to its habitual level, and never for more than a month. Do not be afraid to continue the steroid at full strength in glaucoma patients, as the calming of their trabeculitis may actually help lower pressure. The first recourse should always be to add hypotensive drops and pills to control pressure, reserving an aggressive steroid taper for cases in which this doesn’t work.
Corneal edema. This complication is common and often self-resolving in the first few days or weeks after cataract surgery, but will dramatically affect vision until it clears—usually causing the patient much anxiety. As blur may arise from several factors, it is crucial to rule out a retinal detachment even in cases of evident corneal edema. Immediate post-op corneal edema will come from one of three sources, each treated slightly differently. Examine all layers of the cornea carefully; edema can manifest as microcysts or even bullae at the epithelium, as thickening of the stroma or as folds in the endothelium.
If corneal edema was caused by surgical trauma, it will usually present as stromal thickening and endothelial folds; you may consider adding hypertonic sodium chloride 5% ointment at night if this is severe, but generally this will resolve with time. If the edema is caused by a transient loss of endothelial cell function due to inflammation, you will see 3+ to 4+ cells in the anterior chamber; consider doubling the steroid. If IOPs are greater than 30mm Hg, the hydraulic pressure is likely creating the edema by driving aqueous into the cornea; add timolol or brimonidine to lower IOPs. You may have to employ several tactics simultaneously.7
Any persistent bullae should trigger a phone call to the surgeon. If no improvement is seen after three months, this unfortunate patient will soon be talking to a surgeon about a corneal surgery.
Inflammation. Complex surgeries (e.g., dense cataracts, poor dilations and torn posterior capsules) tend to ignite a vigorous inflammatory response.8 Known cases of uveitis, diabetes or other pre-existing inflammatory diseases are expected to struggle with inflammation. Some patients will even have a post-op uveitis without a difficult surgery.
Your first action should be to search for indicators of endophthalmitis or retained lens material. A hypopyon is a dangerous sign. Having ruled these out, you may simply proceed to dampen the inflammation using stronger or more frequent steroids. If there is a risk of CME, add an NSAID back into the mix. Consider a subconjunctival steroid injection and a surgeon consult if the reaction proves stubborn.
Cataract fragments. Occasionally, lens fragments are inadvertently left in the eye by even the most experienced surgeons. They are most commonly found in the inferior anterior chamber angle or hidden in the capsule equator behind the iris.9 Any unusual level of cells in the anterior chamber should prompt a gonioscopic search of the angle, but a fragment behind the iris will be hard to find.
Lens cortex fragments hydrate and appear fluffy, like cotton; often the eye will melt these away within a few weeks’ time, but, in the meantime, you must control the inflammation carefully. Retained nucleus will look more solid and waxy. This will arouse a greater inflammatory reaction and necessitate a return to surgery for extraction.
You should notify your surgeon of every fragment you notice, even if it is self-resolving.
Refractive surprise. Despite decades of improvements in accuracy, around 26% of patients end up missing their desired refractive target by greater than +/-0.50D.10 If they have undergone LASIK in the past, this number doubles to more than 50%.11 This occurrence, especially if compounded by natural astigmatism, can lead to great disappointment; once again, any seeds of unrealistic expectations will bloom inexorably into post-op discontent. If the preoperative counseling was not performed diligently and you are faced with an unhappy patient, it is best to listen carefully, take a precise refraction and adopt a compassionate attitude. Elective surgical solutions are available but unpalatable: IOL exchange or LASIK.
Toric rotation. Like any lens, toric IOLs depend on precise alignment to work, and not only is this difficult to achieve in the operating room, but the lenses have a 3% chance of rotating during the first month.12 Your best indicators that a rotation may have happened are significant blur and an increase in oddly oriented astigmatism. If your surgeon communicates the desired axis of the implant, you can check this by dilating and looking for the marks indicating the steep axis, but you often do not have this information. A small deviation from alignment that does not greatly affect vision is not a concern.
Any suspicion of a significant rotation should precipitate a return to the surgery center, and quickly; the best time to rotate a toric IOL back into place is within the first few weeks, before the capsule has a chance to fibrose down around the lens.
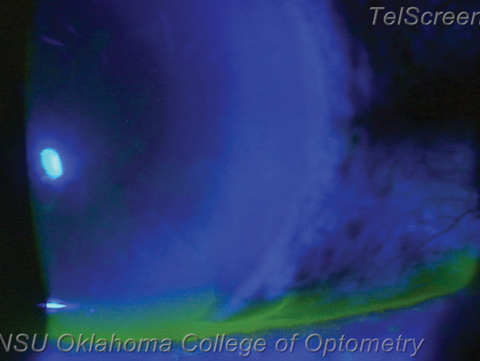 |
| A vigorous wound leak, visible under cobalt blue light as a flow of dark aqueous pushing aside fluorescein. If followed superiorly this leak will reveal its origin at one of the surgical incisions. Photo: Nathan Lighthizer, OD |
Wound leak. If the IOP is less than 8mm Hg or the anterior chamber is shallow, check for a wound leak. Often the patient will complain of significant eye ache. With fluorescein, low IOPs often manifest as waffling of the corneal surface. Instill anesthetic, then gently press a wetted strip of fluorescein around every incision. A leak will show up under cobalt blue light as a stream emerging from the incision: the positive Seidel sign.
If you discover a mild leak, place a tight bandage contact lens, stop or decrease the steroid, notify the surgeon immediately and schedule a follow up the next day. You can even prescribe a topical hypotensive such as brimonidine BID to reduce the hydraulic flow. Wound leak patients must be seen daily until resolution. Mild leaks usually seal spontaneously within a day or two, at which point you can resume the steroid.
If the leak is vigorous, the anterior chamber is so flat as to allow the iris and cornea to touch or the IOP is less than 4mm Hg, call the surgeon and send the patient back immediately; this may need a suture or anterior chamber fill.
Vitreous to the wound. If the posterior capsular tears during surgery, a strand of vitreous can stick to the surgeon’s instruments and during withdrawal be pulled out into an incision. Though invisible when fresh, eventually stray pigment granules stick to the strand, making it easier to spot. The easiest tell-tale sign, however, is a peaked pupil pointing to an incision. This incites a vigorous inflammatory response and requires a quick return to the surgeon, who will likely sever the strand with a Nd:YAG laser.
Cystoid macular edema. This presents overwhelmingly in those with a history of retinal vein occlusion, pre-existing diabetic retinopathy, macular traction from the hyaloid or from an epiretinal membrane, or a posterior capsular tear during surgery. The onset can be from weeks to months after surgery; the patient will typically report initially good vision but later blur.13 Their macula will show a petaloid or honeycomb appearance, sometimes with appreciable elevation. Look for decreased pinhole vision, or the characteristic OCT scan.
CME tends to respond quite well to a combination of a topical steroid and NSAID at their usual dosage. These cases should be seen every two to four weeks; any case that does not improve at each visit merits consideration of a subconjunctival steroid injection or a visit to the retina specialist. If allowed to stagnate, CME can affect vision permanently.
Endophthalmitis. The most feared complication is invasion and infection of the eye by microbes. This can occur during surgery, or later if the wound is slow to heal. Any patient with some combination of significant pain, declining vision, lid edema, severe anterior chamber reaction, hypopyon and inflammatory cells in the vitreous within 72 hours after surgery should be assumed to have infectious endophthalmitis until proven otherwise. Endophthalmitis is rare—from four to 12 per 10,000 eyes in the United States.14,15
Unfortunately, this catastrophic infection does not follow a predictable course and can present in a mild form or even much later with certain microbes. Occasionally, a smoldering uveitis or vitritis is not correctly diagnosed until months later.
You must call the surgery center immediately with any suspicious findings. The next step is often a vitreous tap and culture. The prognosis for confirmed endophthalmitis is poor, with permanently reduced vision often from 20/40 to 20/400 or worse.
Retinal detachments. Eyes without unusual risk factors seem to have no increased risk of retinal tear or detachment after cataract surgery.16 Myopic and lattice degeneration patients, on the other hand, do have an increased risk for up to 10 years after cataract surgery.17 You should caution at-risk patients about the usual symptoms.17
After cataract surgery, patients often regain visual clarity that they have not enjoyed in many years. Watch out for the aforementioned signs, but, in the vast majority of cases, you will get to celebrate a safe and remarkable recovery and enjoy your patients’ satisfaction.
Dr. Kuhn-Wilken is a staff optometrist at Pacific Cataract & Laser Institute, in Tacoma, Wash.
1. Murrill CA, Stanfield DL, VanBrocklin MD. Primary care of the cataract patient. Connecticut: Appleton & Lange; 1994:111-234. |

