 |
History
A 44-year-old Caucasian male reported to the office with a chief complaint of poor night vision. He explained that he had been seen by other eye doctors who had told him he had some “freckles” in his left eye. His systemic and ocular histories were unremarkable and he denied allergies of any kind.
Diagnostic Data
His best-corrected entering visual acuities were 20/20 OU at distance and near. His external examination was normal with evidence of sluggish pupil on the left side. His peripheral confrontation visual field was distorted and constricted in the left eye. The biomicroscopic examination of the anterior segments found normal structures with Goldmann applanation pressures measuring 15mm Hg OU. The pertinent dilated fundus findings are demonstrated in the photographs.
Your Diagnosis
Does the case presented require any additional tests, history or information? What steps would you take to manage this patient? Based on the information provided, what would be your diagnosis? What do you believe is the patient’s most likely prognosis?
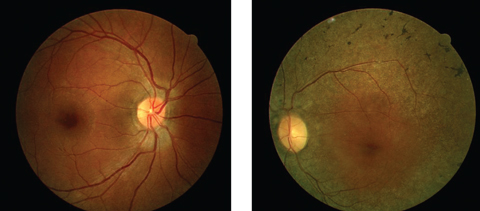 |
| Can these fundus images help point to a diagnosis for this 44-year-old patient suffering from poor night vision? Click image to enlarge. |
Diagnosis
Additional testing should include central and peripheral threshold perimetry and a referral for electrodiagnostic testing (ERG, EOG, dark adaptometry). Cursory blood work was ordered to rule out systemic sources known to produce pigmentary retinopathy. A referral was made to the internist to suggest an electrocardiogram because of documented associations between some pigmentary retinopathies and heart conduction anomalies. Additional historical questions included inquiring if any member of the family had been diagnosed with a similar problem.
The diagnosis in this issue is retinitis pigmentosa (RP). RP is a group of inherited disorders affecting one in 3,000 to 7,000 people.1,2 It is characterized by abnormalities of the photoreceptors (rods and cones) or the retinal pigment epithelium (RPE) of the retina which lead to progressive visual loss.1-14 The predominant symptom is bilateral progressive visual field and acuity loss often proceeding to blindness.1-13 These diseases are transmitted through genetic pedigrees echoing all known modes of inheritance.3 To date, 45 causative genes/loci have been identified in non syndromic RP (for the autosomal dominant, autosomal recessive, X-linked, and digenic forms).3 The most common form of RP is a rod-cone dystrophy.3
Syndromic retinitis pigmentosa (RP) is defined as the disease and its variations with associated groupings of signs, symptoms and systemic findings involving one or more organ systems.3,14 The disease process has many variations with the potential for both early or delayed onset.15 Most patients with RP are diagnosed in the second or third decade of life.5-12 Bardet-Biedl syndrome (BBS) and Usher syndrome (US) are the most prevalent syndrome forms involving RP.1,3,7,11,12,16,17 Together they make up almost a quarter of the patients with RP.12 Bardet-Biedl syndrome is defined by the association of retinopathy, obesity, hypogonadism, renal dysfunction, postaxial polydactyly and mental retardation.1,3,11,12
Usher syndrome is characterized by the combination of congenital or early-onset sensorineural deafness, RP and variable degrees of vestibular dysfunction.11,12,16 Kearns-Sayre syndrome (KS) is a rare disorder consisting of ptosis, limited movement of the eyes and atypical retinal pigmentary changes.7,17 Refsum’s syndrome is characterized by defective peroxisomal alpha oxidation of phytanic acid with clinical features that include retinitis pigmentosa, polyneuropathy, anosmia and hearing loss.12,13,18 Bassen-Kornzweig disease is an autosomal recessive disorder featuring altered lipoprotein metabolism characterized by fat malabsorption, hypocholesterolemia retinitis pigmentosa, progressive neuropathy and acanthocytosis from early infancy.19,20
Patients with retinitis pigmentosa (RP) may present with varying symptoms, which are often gradual and insidious with many patients failing to recognize the advancing manifestations until the disease has progressed significantly.1-24 When symptoms are reported, they initially include difficulty with night vision difficulty (nyctalopia), vision in bad weather as well as loss of peripheral vision.1-8,23 Many patients with RP will also experience visually debilitating photopsias as the disorder progresses.21,22 This phenomenon is believed to represent aberrant electrical impulses from the degenerating retina.21 Central visual acuity is generally not affected until very late stages, although variants have been encountered that cause devastating macular compromise early in the disease course (e.g., X-linked recessive RP, RP inversa).8,23,24 Color vision typically remains intact as long as visual acuity is better than 20/40.8,23 Most patients experience their greatest reductions in central vision between the ages 50 to 80 years.5-13
The ophthalmoscopic appearance of RP finds attenuation of the retinal arterioles, intraneural retinal pigment (bone spicules) in the midperipheral retina and at perivascular locations, thinning and atrophy of the RPE in the mid and far periphery, preservation of macular integrity (except in the condition of RP inversa [macular presentation] and RP sine pigmento [without pigment]), gliotic atrophy of the axons composing the optic nerve (waxy pallor) and choriocapillaris atrophy with increased visibility into the choroid.5-13,24-28 There is a correlation with acquired optic disc drusen in RP.9 In the traditional forms of RP, the appearance and function of the macula and optic nerve remain normal in the early stages of the disease’s development, however, tissue changes in response to the pathology may provoke preretinal gliosis (cellophane maculopathy) which may lead to macular hole, cystoid macular edema, and focal RPE defects.29,30 Additional ophthalmologic findings include ectopic lentis, microspherophakia (Weill-Marchesani syndrome), atypical cataract formation, pigment cells in the vitreous, posterior vitreous detachment and associated vitreous hemorrhage.31-34 Most patients with retinitis pigmentosa are myopic although high hyperopia has been reported.34-36 There is also a correlation with keratoconus.37
The pathophysiology of retinitis pigmentosa is complex.38-46 The common theme of the disease is that it stems from genetic and mitochondrial defects which produce disturbances in the RPE leading to destruction of the photoreceptors’ outer segment disc membranes.2,8,45 The resultant accumulation of metabolic by-products creates disruption of the normal retinal function advancing varying combinations of lipofuscin deposition, retinal gliosis, photoreceptor loss, choriocapillaris occlusion, choroidal atrophy and RPE hyperplasia.2,8,44,45 As the RPE alterations progress, the blood-retina barrier becomes eroded, resulting in intraretinal and subretinal leakage.
There are many recognized forms of retinitis pigmentosa and while most present with similar findings and outcomes, some presentations are atypical.44 Classification of RP may be made on the basis of inheritance pattern (autosomal dominant, autosomal recessive, X-linked, simplex-no family members, multiplex-multiple genes), age of onset (congenital, childhood onset, juvenile onset, adult onset), predominant photoreceptor involvement (rod-cone, cone-rod), or location of retinal involvement (central, pericentral, sectoral, peripheral).5-13,24,44-47
Electrodiagnostic testing remains the gold standard for diagnosis.1-3,24,26,38-40 In RP, both the electroretinogram (ERG) and multifocal electroretinogram (mERG) show significantly diminished red, white, blue and 30 hz flicker waves.35,36 The electro-oculogram (EOG) and dark adaptometry remain as staples in diagnosis and monitoring.5-13,39,40 New testing being evaluated by researchers includes pupillary light reflex. 41-43 Fundus autofluorescence (FAF) which measures the density of lipofuscin granules has emerged as a potential tool as well.25,41-43 Genetic testing can determine the risk of expression in offspring and identify specific gene defects in the affected.1-4,9-25
There is no known treatment to diminish or reverse the progressive retinal dysfunction in retinitis pigmentosa.1-53 Management therefore is three pronged: 1) Prompt diagnosis, 2) Rectify the treatable associated ocular and systemic complications (i.e., refractive error, cataract formation, macular edema, vitreous hemorrhage, hearing loss, dyslipidemia.) and 3) Suggest counseling to maintain quality of life.1-53 While the suspicion of RP is based upon clinical appearance, there are retino-pathological conditions that mimic its distinctive retinopathy. These may include rubella retinopathy, syphilitic retinopathy, cytomegalovirus retinopathies, toxoplasmosis, cancer-associated retinopathy, retinal drug toxicity secondary to thioridazine, chlorpromazine or chloroquine, pigmented paravenous retinochoroidal atrophy and traumatic retinopathy.48-53
Other clinical entities (syphilis49,50 and intestinal polyposis [Gardner’s syndrome]51,52) that cause pigmentary retinopathies must be ruled out with blood work. Serology should be obtained when the diagnosis is unclear or other disorders are suspected. Serology is used to differentiate syndromic forms of RP. Serum phytanic acid testing is used to rule out Refsum disease, electrocardiogram testing is used to rule classic heart manifestations seen in Kearns-Sayre syndrome and a lipid profile with protein electrophoresis can be used to rule out abetalipoproteinemia.7,12,13,17,18,53
Visual field analysis and electrodiagnostic testing along with dark adaptometry should always be obtained to confirm suspected cases.1-3,24,26,36-38 FAF can provide information regarding the integrity of the photoreceptor layer, serving as a secondary instrument for both diagnosis and therapeutic monitoring.41,42
A pedigree can be done to determine the inheritance pattern and to assess risk to offspring.1-4,14,15,44,46-48 Low-vision services are indicated as the disorder affects normal visual function.5,36 Field expansion devices, infrared blocking sun lenses and contrast enhancing filters may all be helpful. Visual field analysis and evaluation for cataract development or macular edema should be performed at least biannually.
The artificial silicon retina (ASR) microchip is a new technology designed to be implanted into the subretinal space to treat vision loss.5 The ASR microchip is a 2-mm-diameter silicon-based device that contains approximately 5000 microelectrode-tipped microphotodiodes.5 It is powered by incident light.5 Visual function improvements have been documented in patients and included unexpected improvements in retinal areas distant from the implant.5
Animal models have led to the development of therapeutic strategies aimed at identifying and curing specific genetic disorders (gene therapy).1-4,44-47 These include growth factors, calcium blocker applications and vitamin supplements. The use of stem or precursor cells are also being inverstigated.5,6,9,54 The newest treatment options include trophic factor therapy, visual cycle inhibitors and cell transplantation.55 A radically different approach has been given the name neural prosthetics ("artificial vision").55 Rewiring of inner retinal circuits are known to occur naturally in retinitis pigmentosa (RP) making researchers believe it is possible to create visually useful percepts by stimulating retinal ganglion cells electrically.55 This has lead to the development of techniques to induce photosensitivity in cells that are not normally light sensitive as well as the development of what is being termed “the bionic retina”.55
Findings in some controlled trials indicate that nutritional interventions, including vitamin A palmitate and omega-3-rich fish, slow progression of disease in many patients.56-58 Patients having retinitis pigmentosa placed on vitamin A therapy with docosahexaenoic acid, 1200 mg/d, demonstrated a slowed the course of disease over the following 2 year period.58 Lutein supplementation of 12 mg/d also has shown promise for slowing loss of midperipheral visual field in nonsmoking adults with retinitis pigmentosa taking vitamin A.57 Supplementation therapy is not free of controversy. Critics point out there is no universally agreed upon regimen and the affects of long-term use remain in question. The literature also suggests that while patients may experience some degree of measurable visual preservation they do not seem to benefit functionally and must be closely medically monitored while on these preparations.44
| 1. Ferrari S, Di Iorio E, Barbaro V, et al. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12(4):238-49. 2. Sahel J, Bonnel S, Mrejen S, Paques M. Retinitis pigmentosa and other dystrophies. Dev Ophthalmol. 2010;47:160-7. 3. Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1(1):40. 4. Koenekoop RK. Why do cone photoreceptors die in rod-specific forms of retinal degenerations? Ophthalmic Genet. 2009;30(3):152-4. 5. Chow AY, Chow VY, Packo KH, et al. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004;122(4):460-9. 6. Dufier JL. Early therapeutic trials for retinitis pigmentosa Bull Acad Natl Med. 2003;187(9):1685-92; discussion 1692-4. 7. Park SB, Ma KT, Kook KH, et al. Kearns-Sayre syndrome -3 case reports and review of clinical feature. Yonsei Med J. 2004;45(4):727-35. 8. Delyfer MN, Leveillard T, Mohand-Said S, et al. Inherited retinal degenerations: therapeutic prospects. Biol Cell. 2004; 96(4):261-9. 9. Obuchowska I, Mariak Z. New approaches towards pathogenesis, diagnosis, natural course and complications of optic disc drusen. Klin Oczna. 2004; 106(1-2):98-101. 10. Ali RR. Prospects for gene therapy. Novartis Found Symp. 2004;255:165-72. 11. Koenig R. Bardet-Biedl syndrome and Usher syndrome. Dev Ophthalmol. 2003;37:126-40. 12. Bamiou DE, Spraggs PR, Gibberd FB, et al. Hearing loss in adult Refsum's disease. Clin Otolaryngol. 2003;28(3):227-30. 13. Hims MM, Diager SP, Inglehearn CF. Retinitis pigmentosa: genes, proteins and prospects. Dev Ophthalmol. 2003;37:109-25. 14. Sahni JN, Angi M, Irigoyen C, et al. Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Curr Genomics. 2011;12(4):276-84. 15. Chang S, Vaccarella L, Olatunji S, et al. Diagnostic challenges in retinitis pigmentosa: genotypic multiplicity and phenotypic variability. Curr Genomics. 2011;12(4):267-75. 16. Yan D, Liu XZ. Genetics and pathological mechanisms of Usher syndrome.J Hum Genet. 2010;55(6):327-35. 17. Fleischhauer J, Njoh WA, Niemeyer G. Syndromic retinitis pigmentosa: ERG and phenotypic changes. Klin Monbl Augenheilkd. 2005;222(3):186-90. 18. Rüether K, Baldwin E, Casteels M, et al. Adult Refsum disease: a form of tapetoretinal dystrophy accessible to therapy. Surv Ophthalmol. 2010;55(6):531-8. 19. Grant CA, Berson EL. Treatable forms of retinitis pigmentosa associated with systemic neurological disorders. Int Ophthalmol Clin. 2001;41(1):103-10. 20. Sani MN, Sabbaghian M, Mahjoob F, et al. Identification of a novel mutation of MTP gene in a patient with abetalipoproteinemia. Ann Hepatol. 2011;10(2):221-6. 21. Lanier KT, Joy JT, Morris RW. Nonclassic retinitis pigmentosa: A challenging clinical diagnosis solved by pedigree analysis and electrodiagnostic testing. Optometry. 2010;81(4):181-7. 22. Bittner AK, Diener-West M, Dagnelie G. Characteristics and possible visual consequences of photopsias as vision measures are reduced in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52(9):6370-6. 23. Jacobson SG, Roman AJ, Aleman TS, et al. Normal central retinal function and structure preserved in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010;51(2):1079-85. 24. Sieving PA. Retinitis pigmentosa and related disorders. In: Yanoff M, Duker JS. Ophtalmology 2nd Ed. Mosby, Philadelphia, 2004: 813-823. 25. Han KH, Kim JW. Electrophysiologic Finding of Retinitis Pigmentosa Inversus and Differential Diagnosis from Peripapillary Choroidal Dystrophy. J Korean Ophthalmol Soc. 1996;37(2):275-283. 26. Zhang Q, Zulfiqar F, Xiao X, et al. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum Genet. 2007;122 (3-4):293-9. 27. Yang C, Liu Y, Lu X, et al. Sporadic bilateral retinitis pigmentosa sine pigmento associated with atypical Peutz-Jeghers syndrome. Can J Ophthalmol. 2010;45(2):184-5. 28. Ferrucci S, Anderson SF, Townsend JC. Retinitis pigmentosa inversa. Optom Vis Sci. 1998;75(8):560-70. 29. Thobani A, Fishman GA. The use of carbonic anhydrase inhibitors in the retreatment of cystic macular lesions in retinitis pigmentosa and X-linked retinoschisis. Retina. 2011;31(2):312-5. 30. Giusti C, Forte R, Vingolo EM. Clinical pathogenesis of macular holes in patients affected by retinitis pigmentosa. Eur Rev Med Pharmacol Sci. 2002;6(2-3):45-8. 31. Ponjavic V, Andréasson S, Abrahamson M, et al. Clinical expression of X-linked retinitis pigmentosa in a Swedish family with the RP2 genotype. Ophthalmic Genet. 1998;19(4):187-96. 32. Watanabe A, Akiyama G, Tsuneoka H. A Case of Retinitis Pigmentosa Requiring Vitrectomy because of Repeated Vitreous Hemorrhage. Case Report Ophthalmol. 2011;2(2):256-61. 33. Hong PH, Han DP, Burke JM, Wirostko WJ. Vitrectomy for large vitreous opacity in retinitis pigmentosa. Am J Ophthalmol. 2001;131(1):133-4. 34. Jethani J, Mishra A, Shetty S, Vijayalakshmi P. Weill-Marchesani syndrome associated with retinitis pigmentosa. Indian J Ophthalmol. 2007;55(2):142-3. 35. Lee SH, Yu HG, Seo JM, et al. Hereditary and clinical features of retinitis pigmentosa in Koreans. J Korean Med Sci. 2010;25(6):918-23. 36. Bogdănici C, Rusu C, Moţoc I, Crăşmaru C. Retinitis pigmentosa--clinical and genetic aspects with low vision. Oftalmologia. 2008;52(2):64-71. 37. Grünauer-Kloevekorn C, Duncker GI. Keratoconus: epidemiology, risk factors and diagnosis. Klin Monbl Augenheilkd. 2006;223(6):493-502. 38. Maiti A, Uparkar M, Natarajan S, et al. Principal components' analysis of multifocal electroretinogram in retinitis pigmentosa. Indian J Ophthalmol. 2011;59(5):353-7. 39. Pojda-Wilczek D. Electroretinogram and electrooculogram in retinal degeneration. Klin Oczna. 1999;101(6):481-5. 40. Kiser AK, Mladenovich D, Eshraghi F, et al. Reliability and consistency of dark-adapted psychophysical measures in advanced eye disease. Invest Ophthalmol Vis Sci. 2006;47(1):444-52. 41. Chen RW, Greenberg JP, Lazow MA, et al. Autofluorescence imaging and spectral-domain optical coherence tomography in incomplete congenital stationary night blindness and comparison with retinitis pigmentosa. Am J Ophthalmol. 2012;153(1):143-154. 42. Liu Y, Liu DN, Meng XH, Yin ZQ. Transient Pupillary Light Reflex in Relation to Fundus Autofluorescence and Dark-Adapted Perimetry in Typical Retinitis Pigmentosa. Ophthalmic Res. 2011;47(3):113-121. 43. Wakabayashi T, Sawa M, Gomi F, Tsujikawa M. Correlation of fundus autofluorescence with photoreceptor morphology and functional changes in eyes with retinitis pigmentosa. Acta Ophthalmol. 2010;88(5):177-83. 44. Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80(7):384-401. 45. Cottet S, Schorderet DF. Mechanisms of apoptosis in retinitis pigmentosa. Curr Mol Med. 2009;9(3):375-83. 46. Clark GR, Crowe P, Muszynska D, et al. Development of a diagnostic genetic test for simplex and autosomal recessive retinitis pigmentosa. Ophthalmology. 2010;117(11):2169-77. 47. Jin ZB, Mandai M, Yokota T, et al. Identifying pathogenic genetic background of simplex or multiplex retinitis pigmentosa patients: a large scale mutation screening study. J Med Genet. 2008;45(7):465-72. 48. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501-26. 49. Vasconcelos-Santos DV, Dodds EM, Oréfice F. Review for disease of the year: differential diagnosis of ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19(3):171-9. 50. Zambon F, Silva FL, Cavalcante AF, et al. Syphilitic retinitis and panuveitis simulating acute retinal necrosis: case report. Arq Bras Oftalmol. 2010;73(3):288-90. 51. Shields JA, Shields CL, Shah PG, et al. Lack of association among typical congenital hypertrophy of the retinal pigment epithelium, adenomatous polyposis, and Gardner syndrome. Ophthalmology. 1992;99(11):1709-13. 52. Cymerys E, Pecold K, Paszkowski J, et al. Retinal changes in patients with familial adenomatous polyposis. Klin Oczna. 2006;108(1-3):70-2. 53. Zamel R, Khan R, Pollex RL, Hegele RA. Abetalipoproteinemia: two case reports and literature review. Orphanet J Rare Dis. 2008;3(7):19. 54. Sahni JN, Angi M, Irigoyen C, et al. Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy.Curr Genomics. 2011;12(4):276-84. 55. Zarbin M, Montemagno C, Leary J, Ritch R. Artificial vision. Panminerva Med. 2011;53(3):167-77. 56. Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368 (9549):1795-809. 57. Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2010;128(4):403-11. 58. Berson E, Rosner B, Sandberg M, et al. Further evaluation of DHA in patients with RP receiving vitamin A treatment. Arch Ophthalmol 2004;122(9):1306-14. |

