 |
History
An 11-year-old Caucasian male reported to the office for a routine eye examination. He explained that his general practitioner wanted him to get his first eye exam after reporting some blur in his left eye over the last month. His systemic and ocular histories were unremarkable and he denied having allergies of any kind.
Diagnostic Data
His best-corrected entering visual acuities were 20/20 OD and 20/25 OS at distance and near. His external examination was normal with no evidence of afferent pupillary defect. The biomicroscopic examination of the anterior segment was normal in every way. Goldmann applanation tonometry measured 15mm Hg OU. The pertinent fundus finding is demonstrated in the photographs and optical coherence tomography.
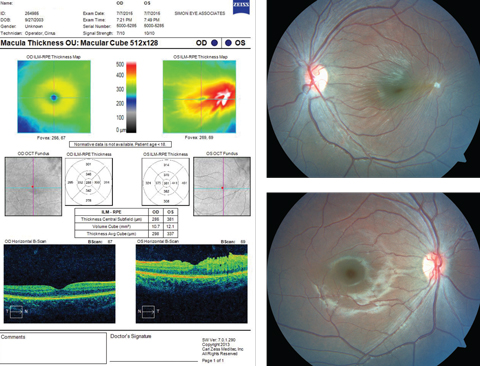 |
| This 11-year-old patient appeared for his first eye exam with blurry vision in his left eye, shown in the bottom fundus image. Can you identify the cause? Click image to enlarge. |
Diagnosis
Additional studies included photodocumentation of the lesion, Amsler grid testing—to understand the effect on function as well as form use in home monitoring—and optical coherence tomography (OCT) to uncover the vitreoretinal etiology and laser interferometry—to understand the maximum potential correctable acuity.
The diagnosis in this issue is vitreomacular tuft with mild macular traction. The vitreous is a clear, gelatinous matrix largely composed of water. Collagen fibers and hyaluronate particles bind the water creating a support structure for the vitreous body.1-5 Condensed collagen fibrils that form the vitreous cortex insert superficially into the internal limiting membrane (ILM) of the retina (basal lamina of the Muller cells).1-5 The vitreous is firmly attached forward of the equator just posterior to the ora serrata, at the vitreous base. This circular band is approximately 4mm wide and serves as the strongest of all vitreal attachments.1-5 Secure attachments are also present at all major retinal vessels, the area of Martgiani around the optic nerve, and the center of the macula.1-5 At the macula, the vitreous is attached in concentric zones centered on the 500μm-diameter foveola.4,5
When condensations of vitreous arise in the absence of vitreous liquification, they are termed tufts. Here, the internal limiting membrane of the retina thickens as incomplete posterior vitreous detachment (PVD) or absence of PVD creates alterations within the vitreoretinal interface to create distortion or retinal striae, or both.6-8 Generically, this condition is referred to as epiretinal membrane (ERM) or macular pucker.8-10 This distortion can affect any macular cellular layer, including surrounding blood vessels.6-10
New evidence suggests that the pathology of ERM, vitreous tufting and vitreomacular traction syndrome is different in adults than they are in children.8 The combination of vitreal and retinal changes in adults results in a decrease of vitreoretinal adhesion throughout the fundus.11,12 Weakened fundus connections promote the migration of vitreous into the subhyaloid space; this causes the vitreous tp displace the surface of the posterior hyaloid from the ILM and form of a posterior vitreous detachment.4,7-10 In the case of vitreomacular traction syndrome (VMTS), the complete separation of the vitreous and retina does not occur, creating tractional forces at the level of the macula.4,7-10
VMTS leads to the symptoms of decreased vision, metamorphopsia, microsia or the photopsia, or a combination.4,5,7 The disturbance among the tight junctions of the retinal vessels and or the tight junctions associated with the zonula occludens junctions of the retinal pigmented epithelium (RPE) can result in thickening and edema of the surrounding neurosensory tissue and corresponding changes in both the appearance and function of the tissues.4,5
VMTS is the tractional elevation of the macula secondary to a partial posterior vitreous detachment.2-5 An epiretinal membrane represents glial cell proliferation between the internal limiting membrane of the neurosensory retina and the posterior hyaloid membrane of the vitreous humor.6,10-12 Glial cells are supportive cells of the central nervous system. ERM may result from a variety of etiologies including trauma, inflammation and vascular damage or may arise idiopathically. Growth, spreading and thickening of ERM can ultimately lead to vitreomacular traction syndrome.2-5,6,10-12 Weakness in the retina and increases in tensile force on the retina can lead to rhegmatogenous tears anywhere traction exists.2-5,6,9-13
Fibrous astrocytes are the primary cell type involved in vitreomacular traction syndrome seem in adults. Fibrocytes and myofibrocytes as well as cellular fragments of the internal limiting membrane, collagen and macrophages may play vital role in the development of VMTS.1,14 In adults, two main pathophysiological aspects of epiretinal fibrocellular proliferation can result in vitreomacular traction syndrome.1,14 The two processes are distinguished based on the presence of cortical vitreous which remains attached to the internal limiting membrane (ILM) following separation of the posterior vitreous.6,7,15,16 In this adaptation, the attachment results in the formation of a cystic edema of the macula with a resultant decrease in vision.6,7 As the pathophysiological outcome of epiretinal membrane evolves, interposed vitreous collagen is remodeled when single cells or a cellular monolayer grows directly onto the ILM.1-16 VMTS can serve as a precursor for macular hole formation.6,7,15-18
Children demonstrate a different pathology in both VMTS and ERM.8 Children have an increased incidence of ERM confluent attachment to the retina and fovea creating increased vessel dragging, typically with foveal sparing. The ERM in children often exhibit “taco” retinal folds as opposed to “ripple retinal folds” seen in adults.8 Further, children possess less fibrillary changes on the inner retina compared with adults.8 Ultrastructural studies via electron microscopy demonstrate increased myofibroblast and collagen with a lower incidence of retinal pigment epithelial cells in their membranes. This difference in histological features may explain the increased frequency of thicker, opaque fibrosis in children.8 Clinical evaluation also suggests a higher incidence of ERM adherence to vessels in children.8 These adherences infiltrate into the posterior vitreous with traction producing subsequent vessel distortion.8 The fibrillary attachments are due to glial cells.8
The patient was referred to a retina specialist who agreed with our diagnosis and, in the absence of substantial lost function, decided to monitor the case with dilated evaluation and serial OCT biannually.
Dr. Gurwood thanks Dr Brad Gardner, Dr. Kevin Brown and Dr. Meagher for their contributions to this case.
| 1. Joyce K, Gurwood, A.S. A look at vitreomacular traction syndrome. Review of Optometry 2011; 148(10): 152. 2.Bainbridge J, Herbert E, Gregor Z. Macular holes: vitreoretinal relationships and surgical approaches. Eye (Lond). 2008;22(10):1301-9. 3. Eun JC, Young JL, Hyo L, et al. OCT-guided Hyaloid Release for Vitreomacular Traction Syndrome. Korean J Ophthalmol. 2008;22(3):169–170. 4. Kleinberg TT, Tzekov RT, Stein L, et al. Vitreous Substitutes: A Comprehensive Review. bSurv Ophthalmol 2011;4(56):300-23. 5. Skeie JM, Mahaian VB. Dissection of the human vitreous body elements for proteomic analysis. J Vis Exp. 2011:23(47)2455-64. 6. Green WR. Vitreoretinal juncture. In: Ryan SJ. Retina. St Louis, Mosby 1989:13-69. 7. Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106(1)624–8. 8. Rothman AL, Folgar FA, Tong AY, Toth CA. Spectral domain optical coherence tomography characterization of pediatric epiretinal membranes. Retina. 2014 ;34(7):1323-34. 9. Byer NE. Cystic retinal tufts and their relationship to retinal detachment. Arch Ophthalmol. 1981;99(10):1788-90. 10. Pop M, Gheorghe A. Pathology of the vitreomacular interface. Oftalmologia. 2014;58(2):3-7. 11. Hirokawa H, Jalk AE, Takahashi M et al. Role of vitreous in independent preretinal macular fibrosis. Am J Ophthalmol 1989;101(1):166-169. 12. Blana SA, Mubhoff F, Hoeller T, et al. Variations in vitreous humor chemical values as a result of pre-analytical treatment. Forensic Sci Int. 2011;210(1-3):263-70. 13. Murakami-Nagasako F, Ohba N. Phakic retinal detachment associated with cystic retinal tuft. Graefes Arch Clin Exp Ophthalmol. 1982;219(4):188-92. 14. Shechtman DL, Dunbar MT. The expanding spectrum of vitreomacular traction. Optometry. 2009 ;80(12):681-7. 15. Gass JDM. Vitreous traction maculopathy. In: Gass JDM. Steroscopic Atlas of Macular Diseases. St Louis, CV Mosby 1987: 678–83. 16. Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86(8):902-9. 17. Reese AB, Jones IR, Cooper WC. Vitreomacular traction syndrome confirmed histologically. Am J Ophthalmol. 1970;69(1):975–7. 18. Hashimoto E., Hirakata A., Hotta K., et al: Unusual macular retinal detachment associated with vitreomacular traction syndrome. Br J Ophthalmol. 1998;82(1):326-27. |

