 |
A 59-year-old Caucasian female presented for a scheduled glaucoma follow-up visit with complaints of decreased vision, foreign body sensation and “uncomfortable” eyes. She was initially seen four years earlier with similar complaints and was diagnosed then as a glaucoma suspect (subsequent testing indicated that she had frank glaucoma, and medication was initiated in both eyes). At that time she was also noted to have generalized ocular surface disease (OSD) with superficial punctate keratitis (SPK), a decreased tear break-up time, a scant tear prism and worsening comfort as the day progressed. She was also found to have anterior and posterior cortical spoking of the crystalline lenses, off the visual axis. Her decreased vision was attributable to the OSD rather than cataracts, and the OSD was addressed appropriately.
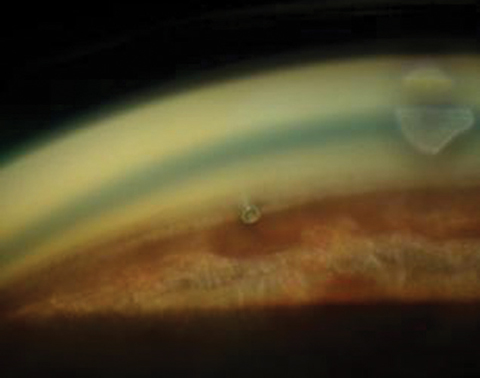 |
| A postoperative cataract patient with an iStent in place. |
Therapies
Over the next several years, her symptoms of irritation fluctuated. Occasionally, her OSD flared, which required topical steroid treatment. Given a glaucoma patient’s propensity to be a steroid responder, it is important to select a steroid with limited ocular penetration, and my go-to drug in these cases is fluorometholone. While this agent is a potent steroid, its potency is limited to the ocular surface, making it an ideal drug to use in glaucoma patients with OSD. But, because of its poor penetrability, it is not an appropriate steroid to use when the anterior chamber is inflamed. Accordingly, she was periodically medicated with the topical steroid to quell her symptoms. Complicating the recovery was the medical management of her glaucoma. While the patient responded nicely to ocular hypotensive agents, their introduction to her eyes every day certainly did not help the OSD complaints.
Examination
At the recent follow-up visit, her entering acuities were 20/50 OD and 20/50- OS. Her ocular medications consisted of unpreserved 0.5% timolol QAM OU, Restasis (cyclosporine, Allergan) BID OU, and unpreserved artificial tears on a PRN basis. Her systemic medications included warfarin, simvastatin and over-the-counter fish oil. She had no known allergies to medications.
A slit lamp evaluation of the anterior segments was remarkable for diffuse SPK in the left eye more so than the right, which, when compared with previous visits, was deemed an average presentation for her. The anterior chambers were clear in both eyes. Through dilated pupils, her crystalline lenses were characterized by progression of the anterior and posterior cortical spoking, now involving the visual axis. We noted minimal nuclear cataract progression in both eyes.
Her cup-to-disc ratios were stable at 0.5 x 0.65 OD and 0.4 x 0.65 OS, each nerve with thinning of the neuroretinal rim inferotemporally. At this visit, Heidelberg retinal tomography (HRT 3) and optical coherence tomography imaging demonstrated stability of the neuroretinal rims, Bruch’s membrane openings, the perioptic retinal nerve fiber layer and the macular ganglion cell scans in both eyes. Her baseline central corneal thicknesses, obtained several years earlier, were 524µm OD and 531µm OS.
Macular evaluations were normal, with only fine retinal pigment epithelium granulation. We noted bilateral posterior vitreous detachments of several years’ duration. The retinal vasculature was characterized by mild arteriolarsclerotic retinopathy consistent with her systemic cardiovascular history, and there were scattered arteriovenous crossing changes, which were deemed stable. Her peripheral retinal evaluations were normal, and the scattered cystoid was stable in both eyes.
Previous visual field studies demonstrated early glaucomatous field loss as obtained by flicker defined form threshold strategy testing, which was not seen on SAP white-on-white perimetry. Repeat testing over several years demonstrated no progression of the field defects. Earlier gonioscopic evaluations demonstrated open angles in both eyes, though ultrasound biomicroscopy imaging demonstrated a slight plateau iris configuration. Her angle studies also have remained stable over the past few years.
Discussion
The patient is presenting with complaints of decreased vision as well as chronic issues with discomfort in both eyes. She was found to have both progressed cataracts as well as SPK on the visual axis. Her glaucoma was stable.
Two important questions must be answered in this case to plot a proper course of action: what’s causing her decreased vision, and what’s her target intraocular pressure (IOP)?
Regarding the question of the decreased vision, this case highlights the overlap of both ocular surface disruption and progressing cataracts, and their effects on vision. Certainly, if there were no cataracts involved, we would see fluctuations in vision commensurate with the degree of OSD findings. These will be seen at various glaucoma follow-up visits, and over time, a clinician will get a feel for what is “normal” for the patient. This, of course, is no different if cataracts were present, but the challenge is to discern how much of the decrease in vision is attributable to the cataract progression vs. how much is related to the OSD. The answer ultimately determines which course to follow—cataract surgery or more aggressive management of the ocular surface disease.
The second question carries even more importance. Remember, the target IOP set for an individual, ideally, is the highest pressure at which no further damage occurs. While IOP reduction may actually be lower than is necessary to meet that threshold, setting a target pressure accomplishes two things: it helps avoid overmedicating the patient, and it gives us a feel for the aggressiveness of the disease process. If a patient’s glaucoma is stabilized with a post-treatment IOP of 15mm Hg, is it really necessary to drive IOP down to 12mm Hg? Certainly, if the treatment involves only one topical glaucoma medication and that results in an IOP of 12mm Hg, we are doing quite well. But if one medication got us to that 15mm Hg threshold, would we really need to add a second medication? I don’t think so (in the hypothetical example here).
While this patient originally presented with glaucoma, we’ve been able to stave off further progression of her disease with a mild-to-moderate reduction in IOP.
In general, I begin glaucoma therapy with a prostaglandin for most patients. And as most patients do, this patient had a significant reduction in intraocular pressure once the prostaglandin was initiated. But, not surprisingly, the addition of any glaucoma medication can aggravate ocular surface disease issues, which is exactly what happened in this case. Ultimately, we settled on unpreserved timolol 0.5% as the glaucoma drug of choice that was tolerable from an ocular surface perspective. While it did not drop IOP as much as other prostaglandins that were prescribed, it did achieve a target level IOP where no further damage was seen.
Given that target IOP in this case does not need to be reduced by 10mm Hg or more and the patient has OSD, we have the perfect scenario to manage both her glaucoma and cataracts with the placement of an iStent (Glaukos) during cataract surgery. iStent placement is estimated to result in IOP lowering of up to 20% from baseline and as much as 10% more than cataract surgery alone provides.1-3 Furthermore, iStents play a significant role in reducing the medication load, which is helpful for patients with concurrent OSD.4,5
With the development of minimally invasive glaucoma surgery techniques, it is more important than ever that the optometrist play an integral role in the cataract surgery planning. The optometrist, after all, has been managing the patient for many years and intimately knows the nuances of their glaucoma. I’ve seen too many instances where optometrists, in their referral to a cataract surgeon, simply state “consider an iStent.” Ultimately, that is a decision best suited for the provider managing their glaucoma; namely, the optometrist. Your cataract surgeon should follow your lead in determining that an iStent is appropriate; if not, perhaps you should be looking for another surgeon.
1. Wellik S, Dale E. A review of the iStent trabecular micro-bypass stent: safety and efficacy. Clin Ophthalmol. 2015 Apr;9:677-84. |

