Serial optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) and threshold visual field analysis, herein referred to as glaucoma progression tests, comprise much of our ability to achieve this task. Both are complemented by statistical software packages designed to enhance this ability. Tonometry, though unsophisticated in what it tells us about the patient, remains essential.
As such, treating glaucoma in 2014 requires clinicians to effectively navigate a variety of testing modalities and analytical software that must be logically integrated into a comprehensive, meaningful clinical picture.
This article looks at practical approaches to reconciling three complex and interrelated components of glaucoma care: IOP assessment, serial RNFL OCT analysis and serial threshold visual field analysis.
Organize IOP Readings
Glaucoma progression tests are only relevant if we can do something about the findings; namely, adjusting the patient’s IOP range through either medical or surgical means. Therefore, it is important to compare any changes in your glaucoma progression tests with the IOP levels during the period in which the changes occurred.
In a busy practice, it is easy to only look at the last couple of IOP readings to gauge the treatment efficacy. However, to successfully treat glaucoma, it is imperative to demonstrate a real IOP lowering effect from the treatments that you have prescribed, and this often requires gathering all IOP data for analysis. Quantifying the IOP reduction from your baseline values will provide context for any changes seen on glaucoma progression tests. This is best achieved by taking the average IOP for each treatment or combination of treatments prescribed.
|
|
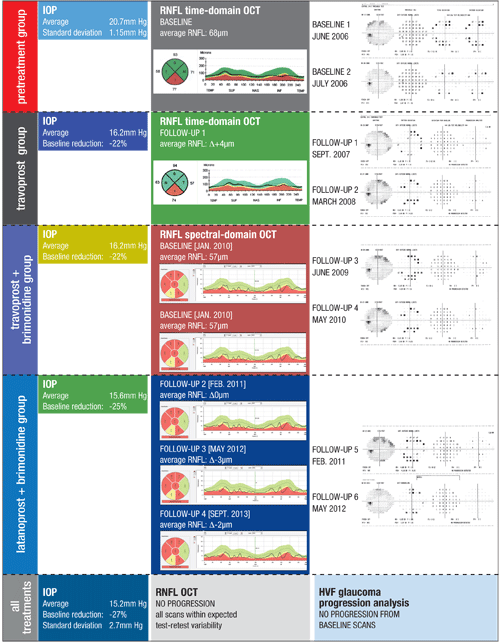
Figure 1. This patient with moderate POAG OS demonstrates the complexity of contemporary glaucoma management. It can take years to establish enough visual fields to accurately determine if progression has occurred. In that time, treatments are added or adjusted, and technologies change, requiring updating of the baseline exams to determine if the therapy is adequate.
Each treatment is demarcated by a block of time to coordinate IOP findings, RNFL OCT scans and GPA for visual fields. Because this patient required multiple medication changes in a relatively short period of time, it was best to use only the pretreatment block and an all-treatments block. Despite a variety of treatments with varying reductions in IOP, this patient’s RNFL OCT and GPA revealed no progression over a seven-year period, thereby meeting the overall management goal. |
Calculating average IOP when analyzing therapeutic response is advantageous in that it has been shown to correlate with the extent of glaucoma progression.1 You may also consider calculating the degree of IOP fluctuation—thought to be a factor in glaucoma progression, although this has been contested.2 It can be estimated using the standard deviation (SD) of IOP (free SD calculators are readily available online) or taking the difference between maximum and minimum values. In studies that did find fluctuating IOP to be a risk factor for glaucoma progression, the SD values were typically greater than 2mm Hg to 3mm Hg. Statistically, estimating the average IOP and SD of IOP becomes more powerful as you accumulate additional IOP measurements.
The process of cataloging IOP readings is very straightforward and only requires that you group all measurements according to the treatments prescribed at that time, if any. For instance, if a patient has been on travoprost monotherapy for four years and I notice they are progressing and add dorzolamide in response, I will establish two treatment groups from which to analyze all of my clinical data (e.g., IOP, RNFL OCT, visual fields): a travoprost group and a travoprost/dorzolamide group. Once this is done, you can calculate the average IOP for each treatment group to assess the extent of IOP lowering.
The pretreatment block of time is the most critical, as this establishes the IOP range that is known to result in a glaucomatous outcome. Therefore, it is recommended that multiple IOPs be taken at different times of day prior to initiating any glaucoma treatments. As a reference, the Early Manifest Glaucoma Trial and Ocular Hypertension Treatment Study, both highly regarded clinical trials, used just two pretreatment IOP measurements from which to judge the efficacy of the treatments studied.3,4
Ideally, recording diurnal IOP measurements on different days is best for assessing the true IOP, as IOP has been shown to fluctuate both spontaneously over time and asymmetrically between the eyes.2,5 Realistically, two to three IOP measurements at different times of day may be sufficient.
Because patient compliance can vary considerably, attempt to grossly quantify the compliance level for each treatment group whenever possible. For instance, if a patient tells me he misses his latanoprost drops about twice a week because he falls asleep prior to instilling the drops, I estimate his compliance to be approximately 70%.
Estimating compliance can be helpful when glaucoma progression tests worsen despite the average IOP meeting the target range. This may suggest that your patient is compliant in the days leading up to their examination, but noncompliant otherwise. Also, when analyzing each treatment group it is important to look for any trending of the IOP values to avoid averaging out potential problems, such as therapeutic tachyphylaxis (i.e., a loss of treatment efficacy over time) or a change in compliance. Always keep in mind that IOP-independent mechanisms may contribute to glaucoma progression, even in the face of low IOP levels.
By grouping and then averaging IOP readings for your various treatments, you will be better prepared to correlate any changes that you encounter in your glaucoma progression tests (see figure 1).
Reconciling OCT Findings
The approach to detecting progressive thinning of the RNFL differs among OCT devices, particularly in the use of software modules to help identify progressive RNFL thinning. Most, if not all, OCT devices assess the RNFL thickness based upon a ring scan centered on the optic disc. In addition to the parapapillary circle scan, some OCT devices also scan the entire area immediately surrounding the optic disc. Irrespective of your OCT’s acquisition and analytical capabilities, it will be important to match the RNFL data with the IOP groups you have established for each glaucoma treatment.
Here are a few ideas on how serial RNFL evaluation can help detect progression as early as possible.
As we move out of the time-domain (TD) and into the spectral-domain (SD) era of OCT acquisition, our ability to produce highly repeatable measurements is vastly improved, as is the ability to detect microstructural changes evident in glaucoma progression.6 With TD-OCT, measurement of the RNFL was limited to the circular line-scan mentioned above.
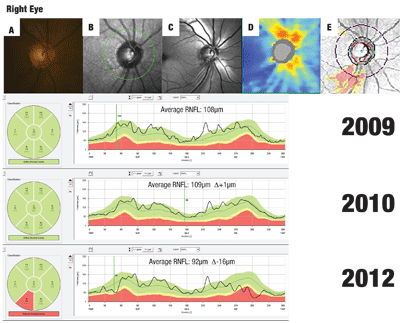
Alternatively, volumetric RNFL assessment of the entire parapapillary region (not capable with a parapapillary circle scan) is now possible with SD-OCT. This method of RNFL analysis yields topographical maps that can assist in defining areas of RNFL depression in the form of wedge defects or generalized thinning—features that are technically difficult to appreciate using funduscopy or photography.7 Figure 2 provides an example of this.
In fact, Leung et al. demonstrated that measuring RNFL thickness using a 3.46mm circumpapillary ring is not as sensitive in identifying progression as using a 6mm x 6mm volumetric grid pattern centered over the optic disc, the acquisition protocol used by the Cirrus HD-OCT (Carl Zeiss Meditec).
The usefulness of constructing an RNFL topographical map around the disc is that it expands the ability to identify widening, deepening or the formation of new RNFL wedge defects, all classic signs of glaucoma progression.7 Although the parapapillary ring scan is still the most commonly used method to assess RNFL thickness, volumetric scans can provide complementary data for detecting glaucoma progression.
Regardless of the RNFL scan type used, detecting RNFL thinning requires good intratest repeatability and point-to-point retinal registration to ensure accurate comparisons between scans, thereby giving you the best chance to determine if progression is occurring.8 Clinical studies show that the average RNFL thickness parameter is useful for demonstrating glaucoma progression.9 This metric is used in guided progression analysis (GPA) software available with Cirrus HD-OCT.
In healthy eyes, the average RNFL thickness should show minimal change over time due to age.
|
|
 |
|
Typically, test-to-test repeatability for SD-OCT ranges from 1µm to 5µm for average RNFL thickness. Therefore, if the average RNFL OCT indices are reduced by greater than 5µm from the baseline values, the clinician should be alerted to possible glaucomatous progression. Evaluating RNFL thickness change by sector and quadrant is also feasible, with the inferior temporal sector and inferior quadrant having greater predictive value for identifying progression.9
Tracking RNFL measurement changes that fall outside the range of expected test-retest variability is termed event analysis.9,10 Simply put, with event analysis you establish two baseline scans at the start of your treatment period; for instance, when starting a patient on a glaucoma medication or switching from monotherapy to combination therapy. You then judge all future RNFL scans to the baseline scans taken at the beginning of the treatment period, knowing that the RNFL values should change minimally if the therapy is effective. This type of analysis can help you determine if progression is present, but does not tell you how fast it is occurring.
To assess the rate of RNFL progression you must perform trend analysis, which can be estimated using OCT GPA. Always be cautious when RNFL thickness measurements change, as they may not always indicate glaucoma progression. Instead, it may be secondary to poor signal strength, misaligned scan, or errors in automated segmentation line placement. To rule out false progression due to artifact in the OCT scan, carefully examine all the parapapillary cross-sectional OCT images and look for areas where the retinal layers appear ill-defined or blurred. These are areas of poor signal strength and can result in deviation of the segmentation lines from the anatomical boundaries of the RNFL, which can influence the average RNFL thickness values.
If the RNFL is progressively thinning based on your serial RNFL OCT analysis, close inspection of the IOP treatment blocks may shed light on the range of IOP that resulted in the changes seen. Although RNFL thinning has been known to be a precursor to functional loss, there may be no concordant change in the visual field status.
Also keep in mind that glaucoma progression can take on different rate patterns, with both a continuous and step-wise pattern having been previously described.9 The former will demonstrate a slow-steady decline, whereas the step-wise pattern will have periods of stability interrupted by periods of progression, typically during intermittent glaucomatous events such as angle closure or compliance issues (see figure 2).
The RNFL is not the only inner retinal structure that shows attenuation commensurate with glaucomatous damage. The ganglion cell layer and inner plexiform layer are also affected, making this a target for assessing glaucoma progression, specifically in the macula. For instance, RTVue (Optovue) OCT assesses the combined thickness of these two layers along with the RNFL, known collectively as the ganglion cell complex (GCC), whereas Cirrus OCT analyzes exclusively the ganglion cell layer and inner plexiform layer.
The utility of ganglion cell OCT imaging in the macula is emerging as a viable clinical tool for assessing glaucoma progression, with some recent evidence suggesting greater sensitivity than RNFL OCT analysis. Specifically, Naghizadeh et al. found that the pattern-based parameters of GCC imaging using the RTVue OCT—which include focal loss volume and global loss volume measurements—may be more effective at this task.11
Although ganglion cell layer OCT analysis adds yet another dimension to glaucoma management, many of the principles used for RNFL OCT analysis also apply. Notably, the test-retest variability ranges from 2µm to 5µm, providing some basis for event analysis.12,13
Visual Field Interpretation
The presence of visual field progression can be detected using a combination of methods, all of which rely on statistical analysis (e.g., glaucoma change analysis, visual field index, GPA). Both GPA and visual field index require customization of the baseline and follow-up visual fields to improve their ability to detect progression and evaluate the success of therapy. Otherwise, the GPA program automatically selects the baseline scans.
Just as in your RNFL OCT progression analysis, visual field analysis also requires that you appropriately set the baseline visual fields according to events that occur during management, such as adding or changing glaucoma drops, recommending glaucoma surgery or the presence of a sustained period of noncompliance.
Despite its ability to recognize visual field progression, GPA can produce misleading results if the baseline visual fields do not correlate with treatment groups, are unreliable, or if the time between the two baseline exams is too long, as this introduces the potential for progression to occur between visual fields.
Choosing the most appropriate baseline visual field is imperative to reveal the effectiveness of the treatments being prescribed. Although resetting the baseline visual field is important to monitor glaucoma progression following a change in treatment, it is not always required. For example, in patients with multiple medication changes, resetting the baselines may actually preclude your ability to accumulate enough follow-up exams to provide meaningful results, as it becomes time prohibitive.
In such cases, it can be better to divide your IOP, RNFL and VF data into a pretreatment group and treatment group, without assessing each individual treatment type (see figure 1). This is usually necessary in patients who have multiple treatment changes over a shorter period of time.
Discordance between structural and functional glaucoma progression is well-recognized. Surprisingly, despite stable OCT RNFL thickness values, up to 35% of patients were shown to develop progressive threshold visual field loss in a study that used TD-OCT.14 Although these results may not correlate directly with the performance of SD-OCT technology, it should be a reminder to the clinician that stable RNFL OCT readings do not always rule out glaucoma progression. As would be expected, progressive OCT RNFL thinning significantly increases the likelihood of future visual field loss.
As such, if a change in glaucoma therapy is made in light of serial RNFL OCT thinning, despite stable visual fields, you should factor in the possibility of a delayed onset of visual field progression when evaluating the efficacy of your new therapy.
The Big Picture
It is easy to become mired in the nuances of glaucoma progression tests and snapshot views of IOP recordings. However, an appropriate analysis of IOP and its relation to glaucoma progression testing can be maintained to preserve the simplicity of glaucoma management.
Scott Anthony, OD, is on staff at the Louis Stokes VA Medical Center in Cleveland, Ohio.
1. The AGIS investigators. Am J Ophthalmol 2000;130:429-440.
2. Leidl M, Choi C, Syed Z, Melki S. Intraocular pressure fluctuation and glaucoma progression: what do we know? Br J Ophthalmol 2014;Epub ahead of print.
3. Leske C, Heijl A, Hyman L, Bengtsson B, et al. Ophthalmolol 1999;106:2144-2153.
4. Gordon M, Beiser J, Brandt J, et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:714-720.
5. Realini T. A prospective, randomized, investigator-masked evaluation of the monocular trial in ocular hypertension or open-angle glaucoma. Ophthalmol 2009;116:1237-1242.
6. Leung C, Chiu V, Weinreb R, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: A comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmol 2011;118:1558-1562.
7. Leung C, Yu M, Weinreb R, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: Patterns of retinal nerve fiber layer progression. Ophthalmol 2012;119:1858-1866.
8. Sehi M, Iverson S. Glaucoma diagnosis and monitoring using advanced imaging technologies. US Ophthalmic Rev 2013;6:15-25.
9. Kotowski J, Wollstein G, Ishikawa H, Schuman J. Imaging of the optic nerve and retinal nerve fiber layer: An essential part of glaucoma diagnosis and monitoring. Surv Ophthalmol 2013: Epub ahead of print.
10. Leung, C. Diagnosing glaucoma progression with optical coherence tomography. Curr Opin Ophthalmol 2014;25:104-111.
11. Naghizadeh F, Garas A, Vargha P, Hollo G. Detection of early glaucomatous progression with different parameters of the RTVue optical coherence tomograph. J Glaucoma 2014;23:195-198.
12. Kotowski J, Wollstein G, Folio L, Ishikawa H, Schuman J. Clinical use of OCT in assessing glaucoma progression. Ophthalmic Surg Lasers Imaging 2011;42:S6-S14.
13. Francoz M, Fenolland J, Giraud J, et al. Reproducibility of macular ganglion cell-inner plexiform layer thickness measurement with Cirrus HD-OCT in normal, hypertensive and glaucomatous eyes. Br J Ophthalmol 2014;98:322-328.
14.Sehi M, Zhang X, Greenfield D, et al. Retinal nerve fiber layer atrophy is associated with visual field loss over time in glaucoma suspect and glaucomatous eyes. Am J Ophthalmol 2013;155:73-82.

