When the U.S. House of Representatives returns from its summer recess, it will consider whether the FDA should resume regulating plano contact lenses as medical devices.
The U.S. Senate has approved a bill (S.172) that would classify all contact lenses as medical devices. The bill would amend the Food, Drug and Cosmetic Act to ensure that all manufacturers and distributors of plano contact lenses are held to the same safety standards as those of prescription lenses.
This bill is important to help keep our children safe, said Sen. Mike DeWine (R-Ohio) when he and Sen. Edward Kennedy (D-Mass.) first sponsored the bill. With non-corrective contact lenses designated as devices, we can prevent needless eye injuries and infections.
The U.S. Senate has approved a bill (S.172) that would classify all contact lenses as medical devices. The bill would amend the Food, Drug and Cosmetic Act to ensure that all manufacturers and distributors of plano contact lenses are held to the same safety standards as those of prescription lenses.
This bill is important to help keep our children safe, said Sen. Mike DeWine (R-Ohio) when he and Sen. Edward Kennedy (D-Mass.) first sponsored the bill. With non-corrective contact lenses designated as devices, we can prevent needless eye injuries and infections.
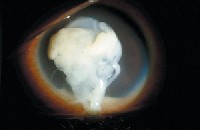 |
| A 14-year-old girl bought colored contact lenses from a video store, without a prescription or instructions and without her parents knowledge or consent. She developed a severe case of Pseudomonas aeruginosa keratitis, as seen here, that required hospitalization and close follow-up for nine months. The ulcer left a visually significant central scar after it healed, and the patient eventually underwent a corneal transplant to restore her vision. Courtesy: Thomas L. Steinemann, M.D. |
The American Optometric Association was very pleased at the final product that was passed by the Senate, said Jon Hymes, the director of the organizations Washington office. It [the bill] will provide for the most appropriate response from the federal government, he says.
The FDA historically treated all contact lenses as medical devices. Then in 2003, the agency determined that plano contact lenses worn solely for cosmetic purposes including colored contacts and decorative lenses, such as WildEyes (CIBA Vision) and Crazy Lenses (CooperVision)were cosmetics.
The FDA historically treated all contact lenses as medical devices. Then in 2003, the agency determined that plano contact lenses worn solely for cosmetic purposes including colored contacts and decorative lenses, such as WildEyes (CIBA Vision) and Crazy Lenses (CooperVision)were cosmetics.
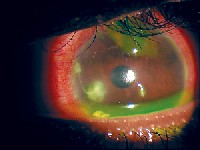 |
| A male in his early 20s bought contact lenses at a flea market and eventually developed this Staph. infection and required hospital admission. Contact lens wear actually was contraindicated in this patient, as his medical history was positive for lupus, for which he took oral prednisone and azathioprine, an immunosuppressant. Autoimmune disease often results in dry eye, and use of an immunosuppressant placed him at higher risk for infection. Courtesy: Thomas L. Steinemann, M.D. |
That meant the FDA could no longer regulate plano lenses as medical devices. The FDA does not review cosmetics for safety or effectiveness before sale to the public. In the case of plano contact lenses, the agency could not set good manufacturing processes for manufacturers or require that consumers be supervised by a health-care professional.
Although the FDA reclassified plano lenses as cosmetics, the agency continued to issue warnings to consumers, especially children and teenagers, about wearing any contact lens without a prescription, proper fitting and instructions from an eye-care professional. The FDA acknowledged that it received reports of patients who suffered corneal ulcers from lenses they bought without a prescription. The ophthalmic literature also reported ocular complications associated with use of contact lenses sold by unlicensed vendors.1
The FDA also said it received reports of decorative contact lenses being marketed and distributed directly to consumers through flea markets, convenience stores, beach shops and the Internet.
A companion bill (H.R. 371), sponsored by Rep. John Boozman (R-Ariz.)the only optometrist in Congressand Rep. Henry Waxman (D-Calif.), is pending in the House of Representatives. Mr. Hymes says the AOA is closely working with Rep. Boozman to make this a priority issue.
An ideal scenario, Mr. Hymes says, would be for the House to consider and pass the Senate bill. If that happens, the legislation would go directly to President Bush, who is expected to sign the bill into law.
Ironically, the House previously passed legislation, but it was not introduced in the Senate. Its very significant that the Senate acted on this prior to recess, especially given that it was the Senate that had not acted on the issue in the previous Congress, Mr. Hymes says. So we think this sends the right message to the House.
Although no timetable has been set for sending the bill to the president, proponents of the bill hope it becomes law by Halloween, a peak period for use of decorative contact lenses. Obviously, if this law is passed, we can start making sure that when Halloween is coming up next time, [the holiday] is a lot safer, says Sarah Hecker, a spokeswoman for Prevent Blindness America. The volunteer eye health and safety organization has designated October as Halloween safety month.
Mr. Hymes agrees. Congressional approval, he says, would help build public awareness about the dangers of using decorative lenses without a prescription.
Although the FDA reclassified plano lenses as cosmetics, the agency continued to issue warnings to consumers, especially children and teenagers, about wearing any contact lens without a prescription, proper fitting and instructions from an eye-care professional. The FDA acknowledged that it received reports of patients who suffered corneal ulcers from lenses they bought without a prescription. The ophthalmic literature also reported ocular complications associated with use of contact lenses sold by unlicensed vendors.1
The FDA also said it received reports of decorative contact lenses being marketed and distributed directly to consumers through flea markets, convenience stores, beach shops and the Internet.
A companion bill (H.R. 371), sponsored by Rep. John Boozman (R-Ariz.)the only optometrist in Congressand Rep. Henry Waxman (D-Calif.), is pending in the House of Representatives. Mr. Hymes says the AOA is closely working with Rep. Boozman to make this a priority issue.
An ideal scenario, Mr. Hymes says, would be for the House to consider and pass the Senate bill. If that happens, the legislation would go directly to President Bush, who is expected to sign the bill into law.
Ironically, the House previously passed legislation, but it was not introduced in the Senate. Its very significant that the Senate acted on this prior to recess, especially given that it was the Senate that had not acted on the issue in the previous Congress, Mr. Hymes says. So we think this sends the right message to the House.
Although no timetable has been set for sending the bill to the president, proponents of the bill hope it becomes law by Halloween, a peak period for use of decorative contact lenses. Obviously, if this law is passed, we can start making sure that when Halloween is coming up next time, [the holiday] is a lot safer, says Sarah Hecker, a spokeswoman for Prevent Blindness America. The volunteer eye health and safety organization has designated October as Halloween safety month.
Mr. Hymes agrees. Congressional approval, he says, would help build public awareness about the dangers of using decorative lenses without a prescription.
1. Steinemann TL, Pinninti U, Szczotka LB, et al. Ocular complications associated with the use of cosmetic contact lenses from unlicensed vendors. Eye Contact Lens 2003 Oct;29(4): 196-200.
Vol. No: 142:9Issue:
9/15/2005

