 |
Genes, which are made up of DNA, are the basic physical and functional units of heredity. The Human Genome Project estimates that humans have between 20,000 and 25,000 genes.1 The DNA sequence of a gene can be altered in a number of ways, resulting in effects that range from common and benign to rare and debilitating.1,2
These gene mutations can be either inherited from a parent and present throughout a person’s life in virtually every cell in the body or acquired at some time during a person’s life and present only in certain cells. These changes can be caused by environmental factors or can occur if an error is made as DNA copies itself during cell division.2,3
Genetic alterations that occur in more than 1% of the population are called polymorphisms.4 These are responsible for many of the normal differences between people such as hair and eye color and blood type. Although many polymorphisms have no negative effects on a person’s health, some may increase the risk of developing certain disorders.4
Research continues to reveal the role genes play in many ocular conditions. Some retinal diseases are caused by a single gene, although these tend to be relatively uncommon, such as Best vitelliform macular dystrophy (generally autosomal dominant). Other, more complex, diseases associated with multiple genetic and environmental factors are relatively common, such as age-related macular degeneration (AMD).4,5
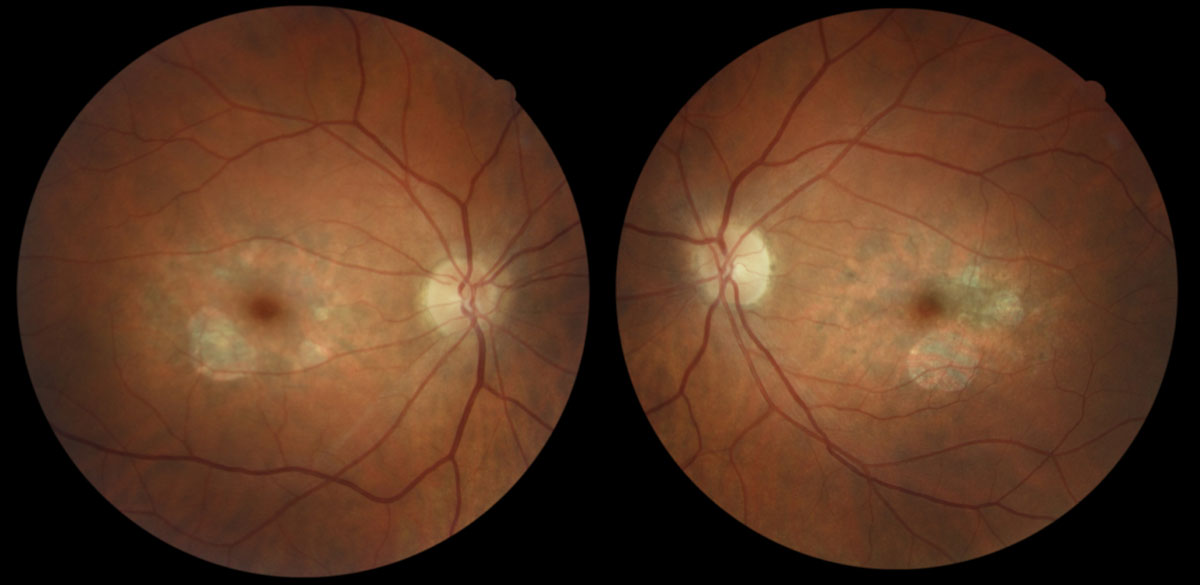 |
| Fig. 1. Parafoveal RPE atrophy with scattered deposits in both eyes of a 43-year-old Caucasian female with butterfly pattern dystrophy. Her best-corrected visual acuity is 20/20 in each eye. Image: Kirsti K. Ramirez, OD. Click image to enlarge. |
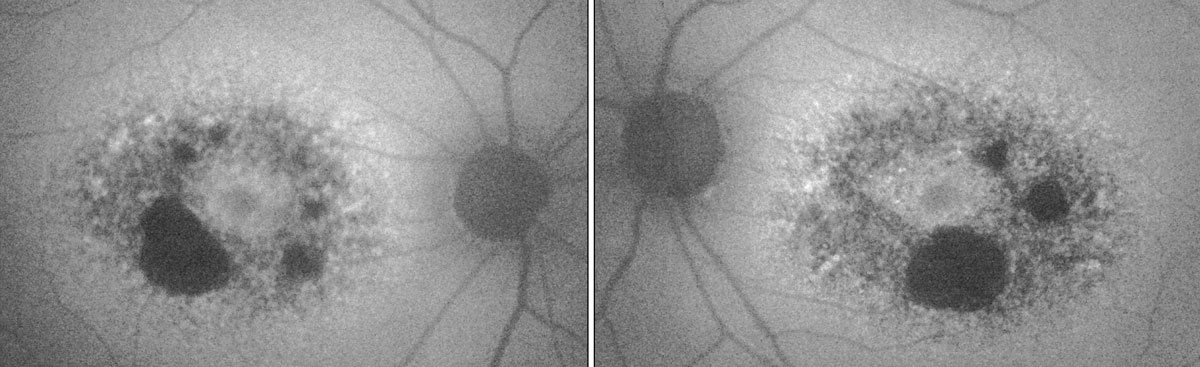 |
| Fig. 2. In the patient’s fundus autofluorescence, the dark areas show RPE atrophy, while the surrounding areas of hyper-fluorescence indicate lipofuscin deposits. Image: Kirsti K. Ramirez, OD. Click image to enlarge. |
Genes and the Retina
In addition to environmental and personal risk factors, research has found that more than 50 gene variants within roughly 35 loci are associated with AMD. The two most extensively studied are complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2).4,5 The latter gene, which shows the strongest association with AMD, encodes a protein that binds to the cell surface and enhances complement activation. Researchers have also genotyped single nucleotide polymorphisms (SNPs) in the CFH and ARMS2 genes.5
The PRPH2 gene, also known as RDS (retinal degeneration slow), encodes a photoreceptor-specific glycoprotein that may play a role in the development and maintenance of photoreceptor outer segment discs. In addition, the gene provides instructions for making the peripherin 2 retina protein, which is essential for the normal function of photoreceptors. Mutation in this gene could mediate pathogenesis by interfering with the integrity of the photoreceptor membrane. Research has identified more than 100 mutations in the PRPH2 gene, many of which cause autosomal dominant retinitis pigmentosa.6
Table 1. Pattern Dystrophies of the RPE6,7
|
PRPH2 mutations also cause pattern dystrophies of the retinal pigment epithelium (Table 1). These dystrophies typically begin in mid-adulthood and are characterized by an abnormal buildup of pigment in cells underlying the retina.6,7 Examples are adult-onset vitelliform macular dystrophy and butterfly-shaped dystrophy (Figures 1 and 2).
Find and Manage Pattern Dystrophies
The phenotypic variation among pattern dystrophies posed a diagnostic challenge. A detailed patient and family history, combined with careful retinal examination, multimodal imaging and genetic testing, should reveal the diagnosis.
All main types of pattern dystrophy have been described in patients with Pseudoxanthoma elasticum. They have also been described in association with myotonic dystrophy, McArdle disease, maternally inherited diabetes, deafness and Crohn’s disease.6,7
Pattern dystrophies can progress, and fundi of older individuals may exhibit atrophic, depigmented lesions extending into the peripapillary region with markedly reduced visual function. Choroidal neovascularization, while possible, is exceedingly uncommon. In general, patients have normal dark adaptation, color vision and full-field electroretinograms. They have intact peripheral fields and may have deficits on electro-oculography.6,7 Although generally not as debilitating as disorders on the retinitis pigmentosa/cone dystrophy spectrum, pattern dystrophies may not necessarily be visually benign.
Although no treatment for pattern dystrophies exists, clinicians should monitor patients for changes during annual eye exams. In addition, clinicians should recommend genetic testing for patients with pattern dystrophies to help pinpoint the dystrophy and provide genetic counseling to the patient and their family members.
Cracking the CodeDNA information is stored as a code made of four chemical bases: adenine (A), guanine (G), cytosine (C) and thymine (T). Human DNA consists of about three billion bases, more than 99% of which are the same in everyone. In humans, genes vary in size from a few hundred DNA bases to more than two million bases. The order, or sequence, of these bases determines the information available for building and maintaining an organism, similar to the way letters of the alphabet appear in a certain order to form words and sentences.3 DNA bases pair up with each other—A with T and C with G—to form units called base pairs. Each base is also attached to a sugar and a phosphate molecule. Together, a base, sugar and phosphate are called a nucleotide. These are arranged in two long strands that spiral in the iconic DNA double helix. The structure of the double helix is like a ladder, with the base pairs forming the ladder’s rungs and the sugar and phosphate molecules forming the vertical rails.1-3 One of DNA’s crucial properties is its ability to replicate. Each strand of DNA in the double helix can serve as a pattern for duplicating the sequence of bases. This is critical when cells divide, because each new cell must have an exact copy of the DNA present in the old cell. The central dogma of molecular biology describes the two-step process, transcription and translation, by which the information in genes flows into proteins: DNA → RNA → protein. Transcription is the synthesis of an RNA copy of a segment of DNA.8 Alleles are forms of the same gene with small differences in their sequence of DNA bases—the key to each person’s unique physical features. In the nucleus of each cell, the DNA molecule is packaged into thread-like structures we know as chromosomes, which contain many genes. A gene mutation is a permanent alteration in a gene’s DNA sequence.1-4 Mutations can affect anywhere from a single DNA base pair to a large segment of a chromosome that includes multiple genes.4 |
|
1. Sadagopan KA, Capasso J, Levin AV. Genetics for the ophthalmologist. Oman J Ophthalmol. 2012;5(3):144-49. 2. Nussbaum RL, Mcinnes RR, Willard HF. Genetics in Medicine. 6th ed. Philadelphia: Saunders; 2004:79-94. 3. US National Library of Medicine. Genetics Home Reference. Help me understand genetics. https://ghr.nlm.nih.gov/primer. Accessed June 4, 2019. 4. Stone EM. Genetic testing for inherited eye disease. Arch Ophthalmol. 2007;125(2):205-12. 5. Schwartz SG, Hampton BM, Kovach JL, Brantley MA. Genetics and age-related macular degeneration: a practical review for the clinician. Clin Ophthalmol. 2016;10:1229-35. 6. Boon CJ, den Hollnader AI, Hoyng CB. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res. 2008;27(2):213-35. 7. Chowers I, Boon CJF. The pattern dystrophies. In: Querques G, Souied E, eds. Macular Dystrophies. Philadelphia: Springer; 2016:11-25. 8. Crick F. The central dogma of molecular biology. Nature. 1970 Aug;227:561-63. |


