A 23-year-old white male, a sailor in the U.S. Navy, complained of a severe headache during his Christmas leave. Specifically, the patient reported severe diffuse frontal pain that progressively worsened over two weeks. His physician referred him to us to determine whether the headache could be visually related.
The patient had no other symptoms, such as nausea, vomiting, transient visual obscurations, tinnitus, double vision or back pain, and he reported no worsening of his headache upon awakening. However, the patient did report seeing lights when he sat up a day earlier. He took either Tylenol (acetaminophen, McNeil) or Motrin (ibuprofen, McNeil) and slept for relief.
The patient had no prior eye examinations, although he did pass the Armed Forces Visual Acuity test before he enlisted. He said that he had excellent vision and had worked at the same job for three years with no visual complaints. He denied any previous ocular trauma, surgery or infection.
The patients medical history was unremarkable. His father has type-2 diabetes mellitus, but his family medical history was otherwise unremarkable. The patient reported no known drug allergies at the time of his visit.
Visual acuity was 20/20 O.U. at distance and near. Pupils were round, equal, responsive to light and without relative afferent pupillary defect. Confrontation fields were normal. Extraocular muscle movements were unrestricted and without double vision or pain. Cover testing and stereopsis were normal.
Color vision testing with Ishihara plates yielded 14/14 O.U. Color saturation test and brightness comparison tests were normal for each eye.
IOP measured 14mm Hg O.D. and 13mm Hg O.S. Slit lamp examination was unremarkable.
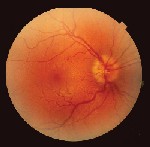
Dilated fundus exam revealed optic nerve head edema with no visible cupping O.U. Retinal blood vessels in both eyes were engorged and tortuous (figure 1). The macula and peripheral retina were within normal limits in each eye.
 |
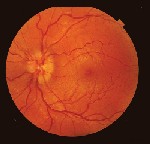 |
| 1. Fundus photos (O.D. left, O.S. right) show optic nerve head edema with no cup visible. Retinal blood vessels in both eyes were engorged and tortuous. |
 |
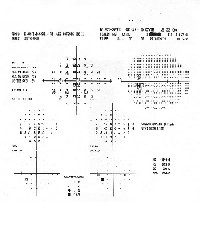 |
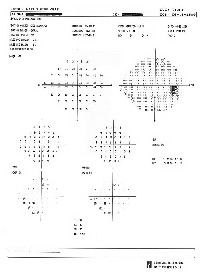
| 2. On 24-2 threshold testing with size III stimulus, (O.D. left, O.S. right), a few points around a somewhat enlarged blind spot show significantly depressed sensitivity values. |
Diagnosis
We tentatively diagnosed bilateral papilledema with secondary enlarged blind spots and headache.
We made an emergency referral for imaging the same day. Non-contrast computed tomography (CT) of the head demonstrated a 3cm long by 3cm wide by 2cm high oval mass in the right frontal lobe. The mass extended into the lateral ventricle with mild dilation of the temporal horns of the lateral ventricles (figure 3). Magnetic resonance imaging (MRI) of the brain (with and without contrast) and an arteriogram confirmed the presence of a right ventricular tumor that had mild hydrocephalous.
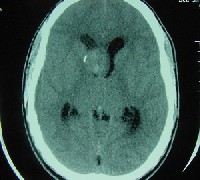 |
| 3. Non-contrast computed tomography scan of the head revealed a 3cm by 3cm by 2cm right frontal lobe mass that extended into the lateral ventricle, with wall calcification on the right side, and which was obstructing the foramen of Monro. This caused deviation of the septum pellucidum to the left as well as deviation of adjacent calcifications to the right. There is evidence of early hydrocephalus with dilation of the temporal horns of the lateral ventricles. |
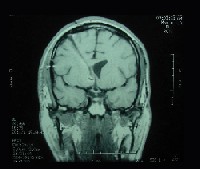
The patient underwent MRI (with and without contrast) two months after the surgery. MRI now showed evidence of a ventriculostomy scar on the right side of the brain but no evidence of a recurrent tumor (figure 4). The patient has taken Dilantin (phenytoin, Pfizer) for seizure prophylaxis since the surgery.
 |
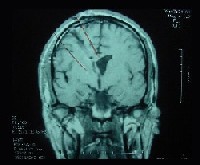 |
| 4. A follow-up brain MRI two months post-op: T1-weighted image without contrast (left) and with gadolinium (right) shows a ventriculostomy scar on the right with no evidence of recurrent tumor. |
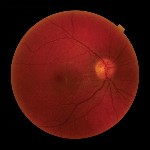
Dilated fundus exam revealed a normal optic nerve head with 0.3x0.3 cup-to-disc ratio in each eye. Rims were pink and distinct, with no edema or pallor. Retinal blood vessels were normal in both eyes. The macula and peripheral retina were within normal limits in each eye (figure 5).
 |
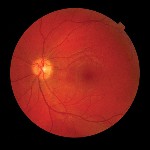 |
| 5. At eight months post-op, the fundus (O.D. left, O.S. right) appeared normal in both eyes. |
 |
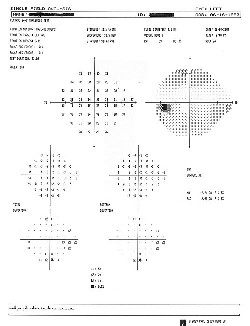 |
| 6. At eight-months post-op, 24-2 threshold testing (O.D. left, O.S. right) showed mild depressed sensitivity in the nasal field O.U. |
Discussion
PXA, first reported in 1979, is an uncommon, slow-growing, astrocytic neoplasm. It usually occurs in children or young adults with no apparent predilection for gender.1 On imaging studies, PXAs often appear well circumscribed, enhance well with gadolinium, and are typically associated with a cyst.2 Like other astrocytic tumors, PXAs can develop in the superficial cortex, cerebellum, spinal cord, posterior hypophysis (pituitary gland) or retina in which astrocytes exist.1,3,4
PXAs are composed of spindle or polygonal mononucleated or multinucleated giant cells that have intracellular accumulation of lipid.3-5 The molecular genetic basis and the tumorigenic mechanism of the disease still remain unclear.
A Japanese study suggests that accumulation of murine double minute 2 (MDM2) without gene amplification might be a major contributor to tumorigenesis of PXA.6-8 Overexpression of MDM2, a protogene that is found in a variety of tumors, inactivates the tumor suppressor p53 in about 10% of human tumors.7 Amplification of MDM2 or increased expression by unknown mechanisms occurs in many tumors.6,7
PXA, a grade 2 astrocytoma according to the World Health Organization (WHO) grading system, is often confused with other tumors. The WHO classification system consists of four grades: grade 1 (pilocytic astrocytoma), grade 2 (diffuse astrocytoma), grade 3 (anaplastic astrocytoma) and grade 4 (glioblastoma multiforme).9
PXA does not have any considerable amount of necrosis, mitotic activity or endothelial proliferation; hence, the prognosis is favorable in most cases.4,6,8 A patient who has PXA without necrosis has an expected median survival of at least 20 years.
By contrast, PXA-like tumors in which there is necrosis have a less favorable prognosis.10 Patients whose tumors have necrosis at initial presentation have a median postoperative survival rate of one year.7
Intracranial tumors produce symptoms primarily by two mechanisms: mass effect (increased intracranial pressure) and destruction of normal tissue.10 Headache is a common presenting symptom. Ophthalmic signs can be important for the diagnosis and management of elevated intracranial pressure. Vision loss, visual field loss, dorsal midbrain syndrome and acute papilledema may occur in advance of ventricular dilation.11 Gastrointestinal upset (e.g., nausea and vomiting), personality changes and slowing psychomotor function may also occur.12
True papilledema that results from increased intracranial pressure may indicate serious neurological disease, so a thorough neurological examination, including emergency CT or MRI, is necessary to rule out intracranial pathologies. Lumbar puncture should follow any abnormal neuroimaging results. Analysis of cerebrospinal fluid may be used to confirm or rule out hemorrhage, infection, tumor or any other intracranial diseases.13
Patients with optic disc edema require regular monitoring with 24-2 or 30-2 threshold visual field testing. Early-phase optic disc edema produces an enlargement of the blind spot. Longstanding optic disc edema can produce secondary optic atrophy, a serious condition that may lead to blindness if left untreated. So, timely treatment is necessary to control intracranial pressure and preserve vision.14 PXAs can recur with or without malignant transformation, so long-term follow-up is necessary.15
Brain tumors, such as pleomorphic xanthoastrocytoma, should be included in the differential diagnosis of papilledema with severe headache. Primary-care optometrists may be the first to encounter clinical manifestations of elevated intracranial pressure that may herald devastating neurological complications. Prompt diagnosis facilitates appropriate referrals, which are vital for management of the condition and its underlying etiologies. Furthermore, an accurate diagnosis and quick referral is key to saving the patients life and preserving his vision.
Dr. Gao and Dr. McGowan are lieutenants in the U.S. Navy and serve as staff optometrists for the Naval Branch Medical Clinic Mayport in Florida.
1. Im SH, Chung CK, Kim SK, et al. Pleomorphic xanthoastrocytoma: a developmental glioneuronal tumor with prominent glioproliferative changes. J Neurooncol 2004 Jan;66(1-2):17-27.
2. Giannini C, Scheithauer BW, Burger PC, et al. Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer 1999 May 1;85(9):2033-45.
3. Kepes JJ, Louis DN, Giannini C. et al. Pleomorphic xanthoastrocytoma. In: Kelihues P, Cavenee WK, eds. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC Press, 2000:52-4.
4. Kepes JJ, Rubinstein LJ, Eng LF. Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer 1979 Nov;44(5):1839-52.
5. Arita K, Kurisu K, Tominaga A, et al. Intrasellar pleomorphic xanthoastrocytoma: case report. Neurosurgery 2002 Oct;51(4):1079-82.
6. Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res 2003 Dec;1(14):993-1000.
7. Ganguli G, Wasylyk B. p53-independent functions of MDM2. Mol Cancer Res 2003 Dec;1(14):1027-35.
8. Matsumoto K, Suzuki SO, Fukui M, Iwaki T. Accumulation of MDM2 in pleomorphic xanthoastrocytomas. Pathol Int 2004 Jun;54(6):387-91.
9. Kleihues P, Cavenee WK. World Health Organization classification of tumors: Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC Press, 2000
10. Pahapill PA, Ramsay DA, Del Maestro RF. Pleomorphic xanthoastrocytoma: case report and analysis of the literature concerning the efficacy of resection and the significance of necrosis. Neurosurgery 1996 Apr;38(4):822-9.
11. Chou SY, Digre KB. Neuro-ophthalmic complications of raised intracranial pressure, hydrocephalus, and shunt malfunction. Neurosurg Clin N Am 1999 Oct;10(4):587-608.
12. Levin VA, Leibel SA, Gutin PH: Neoplasms of the central nervous system. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2001:2100-60.
13. Clinch CR. Evaluation of acute headaches in adults. Am Fam Physician 2001 Feb 15;63(4):685-92.
14. van Roost D, Kristof R, Zentner J, et al. Clinical, radiological, and therapeutic features of pleomorphic xanthoastrocytoma: report of three patients and review of the literature. J Neurol Neurosurg Psychiatry 1996 Jun;60(6):690-2.
15. Korshunov A, Golanov A. Pleomorphic xanthoastrocytomas: immunohistochemistry, grading and clinico-pathologic correlations. An analysis of 34 cases from a single institute. J Neurooncol 2001 Mar;52(1):63-72.

