 An 18-year-old Hispanic female presented for an evaluation at the request of her primary-care physician. She was diagnosed with systemic lupus erythematosus (SLE) four year ago. Also, the patient had a kidney transplant three years earlier and requires dialysis three times a week.
An 18-year-old Hispanic female presented for an evaluation at the request of her primary-care physician. She was diagnosed with systemic lupus erythematosus (SLE) four year ago. Also, the patient had a kidney transplant three years earlier and requires dialysis three times a week.
She takes numerous medications, including folic acid 5mg q.d., pyridoxine 50mg q.d., vitamin B12 1,000mcg injection once per month, Pepcid (famotidine, Merck) 20mg q.d., cyproheptadine 4mg q.d., Phoslo (calcium acetate, Nabi Biopharmaceuticals) 667mg t.i.d., Renagel (sevelamer hydrochloride, Genzyme Corporation) 800mg t.i.d., dapsone 50mg q.d., Neurontin (gabapentin, Pfizer) 200mg b.i.d. and 300mg q.h.s., Plaquenil (hydroxychloroquine, Sanofi-Aventis) 200mg b.i.d., Cozaar (losartan, Merck) 50mg b.i.d. and carvedilol 25mg b.i.d. She also takes Lovenox (enoxaparin, Sanofi-Aventis) 0.3ml/30mg, Bextra (valdecoxib, Pharmacia Corp.) 20mg, Desyrel (trazodone, Apothecon Inc.) 25mg, and kayaxalate. Finally, she takes Epogen (epoetin alfa, Amgen) and Carnitor (levocarnitine, Sigma-Tau Pharmaceuticals Inc.) on Tuesday, Thursday and Saturday prior to dialysis, and Endocet (acetaminophen and oxycodone, Endo Pharmaceuticals) for pain.
Her ocular history was unremarkable. She enjoyed good vision and reported no difficulties with her eyesight. On examination, her uncorrected visual acuity measured 20/20 in each eye.
The patients pupils were equally round and reactive to light, with no afferent defect. Confrontation visual fields were full to careful finger counting O.U., and ocular motility was normal.
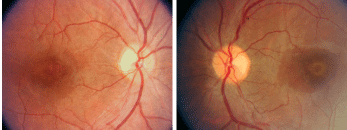
Fundus photographs show peculiar changes in both maculae (O.D. left, O.S. right).
Her Amsler grid findings were normal in each eye, and on color vision testing, she was able to identify 15/15 plates. The anterior segment exam was unremarkable, and her intraocular pressure measured 15mm Hg O.U.
On dilated fundus exam, her optic nerves appeared healthy, with small cups and good rim coloration and perfusion O.U. The vessels were of a normal caliber, and her peripheral retina was unremarkable. The changes seen in both maculae were of interest.
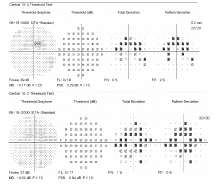
10-2 Humphrey visual fields of our patient show retinal changes (O.D. top, O.S. bottom).
Take the Retina Quiz
1. Which test is highly indicated for this patient?
a. 24-2 threshold visual field.
b. 10-2 threshold visual field.
c. Optical coherence tomography.
d. Fluorescein angiography.
2. How would you describe the changes seen in the maculae?
a. Nonspecific retinal pigment epithelium (RPE) changes.
b. Beaten metal appearance.
c. Neurosensory retinal detachment.
d. Bulls eye maculopathy.
3. What do the findings represent?
a. Lupus retinopathy.
b. Hydroxychloroquine toxicity.
c. Stargardts macular degeneration (SMD).
d. Cone dystrophy.
4. How should she be managed?
a. Instruct the patient to discontinue taking hydroxychloroquine (Plaquenil).
b. Instruct the patient to discontinue dialysis.
c. Educate the patient on the likelihood of central vision loss.
d. Close observation.
For answers, see below.
Discussion
Upon examining both maculae, a yellow-white band, which is an area of RPE depigmentation, can be seen encircling the fovea. The retina appears normal on both sides of the ring. The appearance of the changes suggests a bulls eye maculopathy caused by the use of Plaquenil, which can be toxic to the retina and may cause macular complications.1
Hydroxychloroquine is a quinolone, which is a derivative of a similar drug; chloroquine. Originally, chloroquine was prescribed for the treatment and prophylaxis of malaria. Then, rheumatologists used it to treat rheumatoid arthritis, systemic/discoid lupus erythematosus, and other connective tissue and inflammatory disorders. Yet, some patients on chloroquine developed a bulls eye maculopathy.
Hydroxychloroquine is far less toxic to the retina than chloroquine and has widely replaced chloroquine, except for those who travel where malaria is endemic. Retinal toxicity from hydroxychloroquine is quite rare, considering the number of people who use Plaquenil. For those patients who develop retinal toxicity, permanent vision loss may result, even after stopping medication.
Evidence suggests that retinal toxicity incidence depends both on dosage volume and duration; the majority of reported cases have occurred at dosages greater than 6.5mg per kilogram (of body weight) per day in patients who used Plaquenil for more than five years.2
The typical dosage of Plaquenil is either 200mg or 400mg per day. The use of 200mg per day puts anyone who weighs less than 68 pounds at risk for retinal toxicity, and 400mg per days puts anyone less than 135 pounds at risk.4 So, 200mg of Plaquenil per day is a safe dosage for almost all adults, unless they are particularly diminutive or have other risk factors, such as kidney failure or liver disease.4-5
In 2002, the
For patients in the low-risk category, the AAO recommends that no additional special ophthalmologic testing is needed for five years. However, an annual eye examination is recommended for patients in the high-risk category.
All patients who use hydroxychloroquine should have a dilated fundus exam and a visual field test by Amsler grid or 10-2 central threshold visual field within the first year after starting the medication.2
If toxicity is suspected or documented, immediately notify the patients prescribing physician and recommend that he or she discontinue the medication.2 Unfortunately, there may not be any better alternatives, as many comparable medications may cause even worse toxicity than hydroxychloroquine.
We see our patient annually and perform Amsler grid testing, 10-2 central threshold visual fields, color vision testing and a dilated fundus exam at each visit. We may see higher-risk patients more frequently and perform the same battery of tests each time, especially if toxicity is suspected. We also take a baseline fundus photograph when the patient begins hydroxychloroquine, and periodically thereafter.
Our patient was seen before the AAO guidelines were released. However, even without these guidelines, it was evident that she was at a higher risk of developing retinal toxicity due to her relatively low body weight and kidney failure. Remarkably, her visual acuity never dropped below 20/20, and her color vision remained normal. We were able to detect a ring scotoma on 10-2 central threshold visual fields. Also, due to her relatively poor health, she was not a good test taker, which made it difficult to interpret and depend on the visual field results. We gave our patient a report that explained how retinal toxicity is associated with Plaquenil use. We also related our findings to her rheumatologist, and he instructed the patient to discontinue the medication.
1. Easterbrook M. Detection and prevention of maculopathy associated with antimalarial agents. Int Ophthalmol Clin 1999 Spring;39(2):49-57.
2. Marmor M, Carr R, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the
3. Roque MR, Roque BL, Foster SC. Chloroquine/Hydroxychloroquine toxicity. Availble at: www.emedicine.com/oph/topic245.htm (Accessed May 13 2008).
4. Easterbrook M. Screening for antimalarial toxicity: current concepts. Can J Ophthalmol 2002 Oct;37(6):325-8, 331-4.
5. Torbit, J. Plaquenil toxicity detected without bulls eye maculopathy. Available at: www.opt.indiana.edu/ce/plaq/risks.htm (Accessed May 13 2008).
Retina Quiz Answers: 1) b; 2) d; 3) b; 4) a.

