 A 79-year-old white male with pseudoexfoliative glaucoma
(PXG) presents for his regularly scheduled progress evaluation. He has no
visual complaints, and he reports that he is compliantly using Travatan Z
(travoprost, Alcon) at bedtime and Trusopt (dorzolamide, Merck) twice a day in each
eye.
A 79-year-old white male with pseudoexfoliative glaucoma
(PXG) presents for his regularly scheduled progress evaluation. He has no
visual complaints, and he reports that he is compliantly using Travatan Z
(travoprost, Alcon) at bedtime and Trusopt (dorzolamide, Merck) twice a day in each
eye.
His intraocular pressure measures 16mm Hg O.U., which is the highest IOP desired for this patient, given his thin corneas and advanced optic disc and visual field damage. As expected, a dilated lens examination reveals a classic bull’s-eye appearance of exfoliative material on each lens. Additionally, there are significant peripupillary iris transillumination defects, and there is a high degree of pigmentation of the trabecular meshwork visible via gonioscopy.
This patient’s PXG seems well controlled, as evidenced by his IOP, which is therapeutically reduced from the untreated 30s to the treated mid-teens. Additionally, his discs and fields have not progressed since treatment began. Is the standard therapy used here enough, or is there something else that can be done non-invasively to positively impact this patient’s disease? The answer to the latter is yes. This month, we look at an old standby for PXG: pilocarpine.
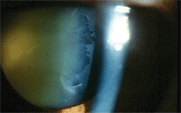 |
| This patient demonstrates
the classic appearance
of pseudoexfoliative material accumulating on
the lens surface.
|
Pseudoexfoliation occurs in 5% of older Americans, though the true overall prevalence may be underestimated; as many as 15% of cases may be missed clinically.1,2 Pseudoexfoliative glaucoma is rarely found in patients younger than 50 years of age.3 Patients with pseudoexfoliation present with a fine, flaky material on the anterior lens capsule that may initially be seen at the pupillary margin. This material may be visible in an undilated state, but can easily be missed. Over time, it coalesces into the characteristic bull’s-eye pattern typically seen in pseudoexfoliation syndrome. This pattern is usually only observable when the patient’s pupil is dilated.
Another hallmark of the condition is pigment loss from the pupil margin with subsequent deposition on anterior chamber structures.4 This leads to increased transillumination of the iris at the pupillary margin—peripupillary transillumination defects. Pseudoexfoliative material can lead to the formation of posterior synechiae, as this material can be quite “sticky.” Early cases may manifest as heavy pigment deposition on the lens surface in a radial pattern from broken synechiae.
Pseudoexfoliation involves the production and accumulation of an abnormal fibrillar extracellular material within the anterior chamber of the eye.5,6 The accumulated material consists of a fibrillar component and an amorphous component, though the exact chemical composition remains unclear.7-11 It appears that the material is abnormal basement membrane that becomes deposited on the anterior lens capsule, iris surface, and trabecular meshwork.7-11 Due to accumulation of material at the pupillary margin, there is increased lenticular apposition with the iris and subsequent erosion of iris pigment as the pupil dilates and constricts. This iridolenticular friction leads to the increased iris transillumination and deposition of pigment granules on the corneal endothelium, iris surface and trabecular meshwork, similar to that seen in pigment dispersion syndrome.
The primary cause of IOP elevation appears not to be physical blockage of the trabecular meshwork by pigment granules, but the phagocytosis of accumulated pigment and pseudoexfoliative material by the trabecular cells and Schlemm’s canal cells with subsequent degenerative changes of Schlemm’s canal and trabecular meshwork tissues.12 In patients with PXG, trabecular meshwork pigmentation correlates more strongly with presenting IOP than the amount of pseudoexfoliative material on the anterior lens capsule.13
Management of PXG
Pseudoexfoliative glaucoma is medically treated in the same manner as primary open-angle glaucoma (POAG)—with topical beta-blockers, topical carbonic anhydrase inhibitors, prostaglandin analogs and alpha adrenergic agonists. However, the IOP level in PXG is typically higher than in POAG and is more difficult to bring down. Typically, a greater amount of medical therapy is needed to control patients with exfoliative glaucoma vs. POAG patients.14-16 Is there any additional medical therapy that can help not only to stabilize IOP in these patients, but perhaps also to alter the actual pathogenic process? In other words, if pigment liberation seems to be the factor most implicated in the IOP process, then would it help to reduce the degree of pigment liberation? One viable way to accomplish this reduction would be to reduce the iridozonular friction created by the iris moving across the lens laden with material. This is easily accomplished through the use of low concentrations and low dosing of pilocarpine. Although pilocarpine has a relatively short duration of pressure-lowering action and is typically prescribed q.i.d. to lower IOP, its miotic effect seems to linger. Once-daily dosing of pilocarpine (at bedtime, generally) can achieve this aim.
The goal here is not so much IOP reduction, but rather reduction of iris mobility. Robert Ritch, M.D., in his Optometric Glaucoma Society 2008 Honoree Lecture, advocated this once-daily therapeutic approach, and most patients readily accept it.17 Local effects from the ciliary body constriction, such as brow ache, can also be avoided by dosing at bedtime.
To that end, we prescribed our patient pilocarpine 2%, to be dosed O.U. at bedtime, with specific instructions to wait several minutes between administration of pilocarpine and Travatan Z to avoid washout. When he returned for his regular follow up three months later, he reported no difficulties with the new medication. His vision was unchanged, and he never experienced head or brow ache. Additionally, his IOP now measured 13mm Hg O.U. Then again, there appeared to be no difference in the amount of trabecular meshwork pigment, and the improved IOP could have been a coincidence. Most likely, it may be quite some time before any benefit is seen with this adjunctive therapy. Possibly, pilocarpine may not reduce the amount of pigment being liberated.
Still, the reasoning behind pilocarpine usage is sound, and low dosing may achieve the desired goals without inducing the adverse effects usually associated with its use. Adjunctive use of pilocarpine has little risk of harming the patient. Pilocarpine is a very inexpensive medication, so its use likely won’t financially overburden a patient. More clinicians seem inclined to try pilocarpine again. So, for patients with PXG, we say, “Hit the pilo before you hit the pillow.”
1. Hiller R, Sperduto RD, Krueger DE. Pseudoexfoliation, intraocular pressure and senile changes in a population based survey. Arch Ophthalmol. 1982 Jul;100(7):1080-2.
2. Krause U, Tarkkanen A. Cataract and pseudoexfoliation: a clinicopathological study. Acta Ophthalmol (Copenh). 1978 Jun;56(3);329-34.
3. Kozobolis VP, Papatzanaki M, Vlachonikolis IG, et al. Epidemiology of pseudoexfoliation in the island of Crete (Greece). Acta Ophthalmol Scand. 1997 Dec;75(6):726-9.
4. Vesti E, Kivelä T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res. 2000 May;19(3):345-68.
5. Layden WE, Shaffer RN. Exfoliation syndrome. Trans Am Ophthalmol Soc. 1974 Nov;78(5):835-41.
6. Mudumbai R, Liebmann JM, Ritch R. Combined exfoliation and pigment dispersion: an overlap syndrome. Trans Am Ophthalmol Soc. 1999;97:297-321.
7. Amari F, Umihira J, Nohara M, et al. Electron microscopic immunohistochemistry of ocular and extraocular pseudoexfoliative material. Exp Eye Res. 1997 Jul;65(1):51-6.
8. Kubota T, Schlötzer-Schrehardt U, Inomata H, Naumann GO. Immunoelectron microscopic localization of the HNK-1 carbohydrate epitope in the anterior segment of pseudoexfoliation and normal eyes. Curr Eye Res. 1997 Mar;16(3):231-8.
9. Naumann, GO, Schlötzer-Schrehardt, U, Kuchle M. Pseudoexfoliation for the comprehensive ophthalmologist. Ophthalmology. 1998 Jun;105(6):951-68.
10. Lis GJ. Pathogenesis and histopathology of pseudoexfoliative lesions. The eyeball disease or ocular manifestation of a generalized process? Przegl Lek. 2006;63(7):588-92.
11. Ludwisiak-Kocerba L, Hevelke A, Kecik D. Pseudoexfoliation syndrome—etiopathogenesis and clinical course. Klin Oczna. 2006;108(1-3):82-6.
12. Ritch R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003 May;22(3):253-75.
13. Shuba L, Nicolela MT, Rafuse PE. Correlation of capsular pseudoexfoliation material and iridocorneal angle pigment with the severity of pseudoexfoliation glaucoma. J Glaucoma. 2007 Jan;16(1):94-7.
14. Konstas AG, Stewart WC, Stroman GA, Sine CS. Clinical presentation and initial treatment patterns in patients with exfoliation glaucoma versus primary open angle glaucoma. Ophthalmic Surg Lasers. 1997 Feb;28(2):111-7.
15. Ritch R. Initial treatment of exfoliative glaucoma. J Glaucoma. 1998 Apr;7(2):137-40.
16. Konstas AG, Lake S, Maltezos AC, et al. Twenty-four hour intraocular pressure reduction with latanoprost compared with pilocarpine as third-line therapy in exfoliation glaucoma. Eye. 2001 Feb;15(Pt 1):59-62.
17. Konstas AG, Tsironi S, Ritch R. Current concepts in the pathogenesis and management of exfoliation syndrome and exfoliative glaucoma. Compr Ophthalmol Update. 2006 May-Jun;7(3):131-41.

