 |
Q: I have a patient who has had well-controlled COAG for years. The pressure went up dramatically and fairly suddenly after a month on a nasal spray. Could this be related?
A: “Your patient’s sudden and dramatic increase in pressure can most certainly be related to her-or-his use of nasal spray,” says Kristen Thelen, OD, of Advanced Eye Center in Gainesville, GA. In 2013, Nasacort (triamcinolone acetonide, Sanofi-Aventis) became the first over-the-counter steroid nasal spray available in the United States.
“We have known for years that steroids of many forms can cause an increase in outflow resistance of the trabecular meshwork, thus leading to an increase in intraocular pressure (IOP),” says Dr. Thelen. One study showed that inhaled nasal glucocorticoids, when used at high doses and regularly for three or more months, put patients at a one-and-a-half times increased risk for ocular hypertension and glaucoma, she says. The study also suggested that certain populations are at higher than average risk for developing a steroid response, and glaucoma patients in particular.1
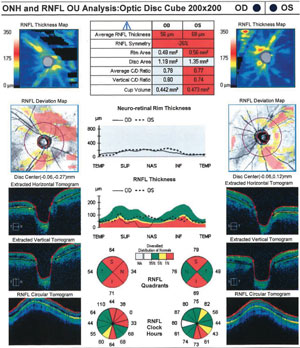 | |
| Cirrus OCT analysis following IOP spike shows decline in retinal nerve fiber layer thickness. |
Dr. Thelen describes a recent case: A 59-year-old white male presented with increased intraocular pressure following two months of using Nasacort nasal spray. The patient suffered from fall allergies and started taking over-the-counter triamcinolone acetonide twice a day. Intraocular pressure increased from 19mm Hg and 14mm Hg to 40mm Hg and 39mm Hg in the patient’s right and left eyes, respectively. The patient was using Combigan (brimonidine tartrate/timolol maleate ophthalmic solution 0.02%/0.05%, Allergan) OU BID and Xalatan (latanoprost ophthalmic solution 0.005%, Pfizer) OU QHS for his glaucoma, which had been well controlled for roughly 30 years.
The patient was treated with 500mg of Diamox sequels (acetazolamide ER, Duramed Pharmaceuticals) PO BID and instructed to discontinue use of the triamcinolone acetonide indefinitely. Within two weeks, the patient’s intraocular pressure dropped to 19mm Hg and 15mm Hg OD OS. Upon discontinuation of Diamox, the patient’s intraocular pressure increased. A second round of Diamox was started and the patient’s IOP decreased permanently to normal levels.
Dr. Thelen advises doctors to look into a patient’s history for possible risk factors prior to administration of steroidal nasal sprays.
“Some things to focus on are your patient’s stage of glaucoma, how many glaucmatous risk factors are present, including age, ethnicity and family history, and the duration and frequency at which your patient was using the nasal spray.” All these factors can play a part when trying to determine the likelihood of a steroid nasal spray resulting in an IOP spike, says Dr. Thelen.
Dr. Thelen says that managing acute elevations in intraocular pressure is nothing to stress over. “Find reassurance knowing that IOP spikes are treated no differently than glaucoma—the spike will usually go away when discontinuing the steroid.” Dr. Thelen recommends closely monitoring this patient until intraocular pressure returns to normal.1. Garbe E, Lelorier J, Boivin JF, Suissa S. Inhaled and Nasal Glucocorticoids and the Risks of Ocular Hypertension or Open-angle Glaucoma. JAMA. 1997 Mar 5;277(9):722-7.

