Cornea ReportCheck out the other feature articles in this month's issue: My Patient has Recurrent Corneal Erosion...Now What? |
The conditions ODs dread managing are those that highly impact patients’ lives but for which few treatments truly succeed. Keratoconus was one of them, because management frequently involved eyeglass remakes, several contact lens prescriptions, rigid contact lenses that displaced and caused discomfort and, for the optometrist, historically inadequate third-party reimbursement.
But in the past two decades, several new options have turned this frustrating treatment paradigm on its head. Today, Intacs (Addition Technologies) and corneal crosslinking (CXL) are new medical strategies, in addition to advanced hybrid and scleral contact lenses. Keratoconus is now an opportunity more than it is an obstacle. ODs can make the most of the opportunity by updating their understanding of the disease and its many therapies.
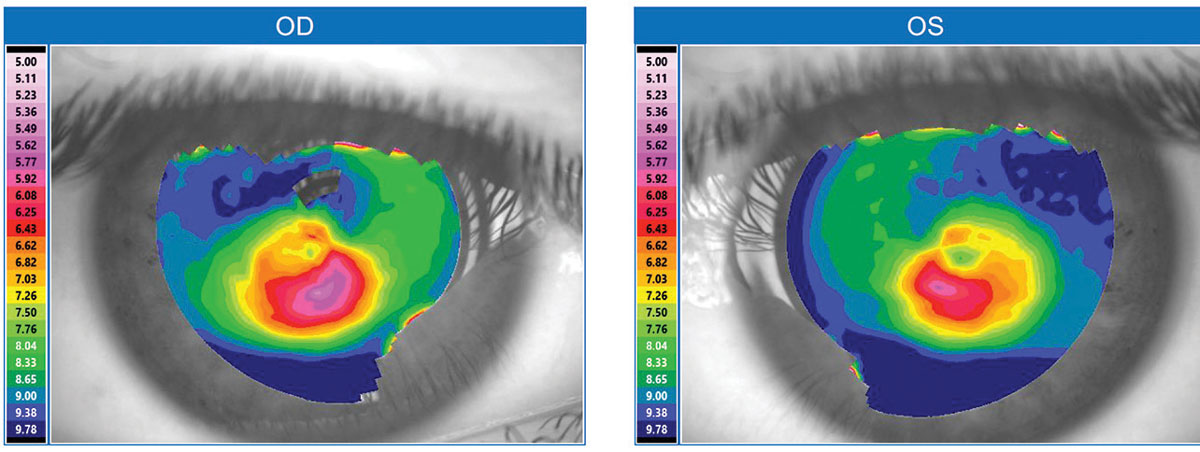 |
| Fig. 1. This topographical map is typical for a keratoconus patient. Click image to enlarge. |
New Attention, Old Disease
Keratoconus is a well-recognized contraindication for undergoing LASIK because excimer ablation can further weaken and destabilize the keratoconic cornea, making vision much worse. Nonetheless, the rise of LASIK has indirectly benefited those with keratoconus. Since the first FDA approval of an excimer laser for LASIK in 1999, increased screenings for LASIK candidacy have identified many patients who were previously unaware of their keratoconus. Technologies such as corneal topography, Scheimpflug imaging and optical coherence tomography (OCT) have also helped identify more keratoconus patients (Figure 1). Unlike corneal topography, which only measures the anterior cornea surface, Scheimpflug imaging and OCT can measure both the anterior and posterior corneal surfaces and perform pan-corneal pachymetry. These measures are valuable for diagnosis because keratoconus often demonstrates abnormal anterior and posterior corneal curvatures in conjunction with corneal thinning. With these improved screenings, the known population with keratoconus has grown, and mild “forme fruste” cases that formerly escaped diagnosis are more readily detected. Previous estimates suggest one in 1,800 has keratoconus, but a 2017 population study in the Netherlands suggests it is closer to one in 375.1,2
Although previous research suggested unilateral keratoconus was possible, today’s diagnostics tools show these are in fact highly asymmetric cases. A thorough clinical exam often demonstrates tell-tale abnormalities of keratoconus in the patient’s “good” eye.3 Furthermore, patients with pellucid marginal degeneration (PMD) now add to the growing list of identified keratoconus patients. Experts agree that keratoconus and PMD are the same underlying disease but with different clinical presentations.3 Still, some researchers believe PMD is a different disease—patients with “crab-claw” corneal topographies on axial view represent inferior keratoconus much more frequently than PMD, for example.4
The greater awareness of keratoconus has spurred significant development of new and improved interventions that augment the established legacy treatments.
 |
| Fig. 2. Silicone hydrogel skirt UltraHealth hybrid lens. Image by SynergEyes. |
Therapy Standbys: PK and Corneal RGPs
During the 19th century, researchers proposed some radical surgical interventions for keratoconus, including translocation of the pupil away from the corneal apex, incarceration of the iris in a corneal incision to create a stenopaic slit pupil, and chemical and thermal cautery of the cornea—none of which took hold.5 It wasn’t until 1936 when Castroviejo performed the first penetrating keratoplasty (PK) for keratoconus in New York that the role of surgery took form.6 Since then, PK has been the dominant, albeit end-of-the-line, surgical intervention. Many patients undergoing PK experience a protracted recovery, still need to wear rigid surface contact lenses to obtain functional vision and often require a chronic maintenance dose of topical corticosteroids to prevent graft rejection.
Restoring vision in keratoconus has revolved around corneal rigid gas permeable (RGP) contact lenses, with glasses and soft contact lenses serving a smaller number with mild disease. Although corneal RGPs do not retard disease progression, they serve to improve vision, a task often undermined by limited contact lens tolerability. Among RGP wearers in the Collaborative Longitudinal Evaluation of Keratonus study, 27% reported lens discomfort, excluding participants who had already discontinued RGP lens wear before study enrollment.7 Other patients report problems with corneal RGP lenses ejecting and dislodging, as well as handling difficulty.
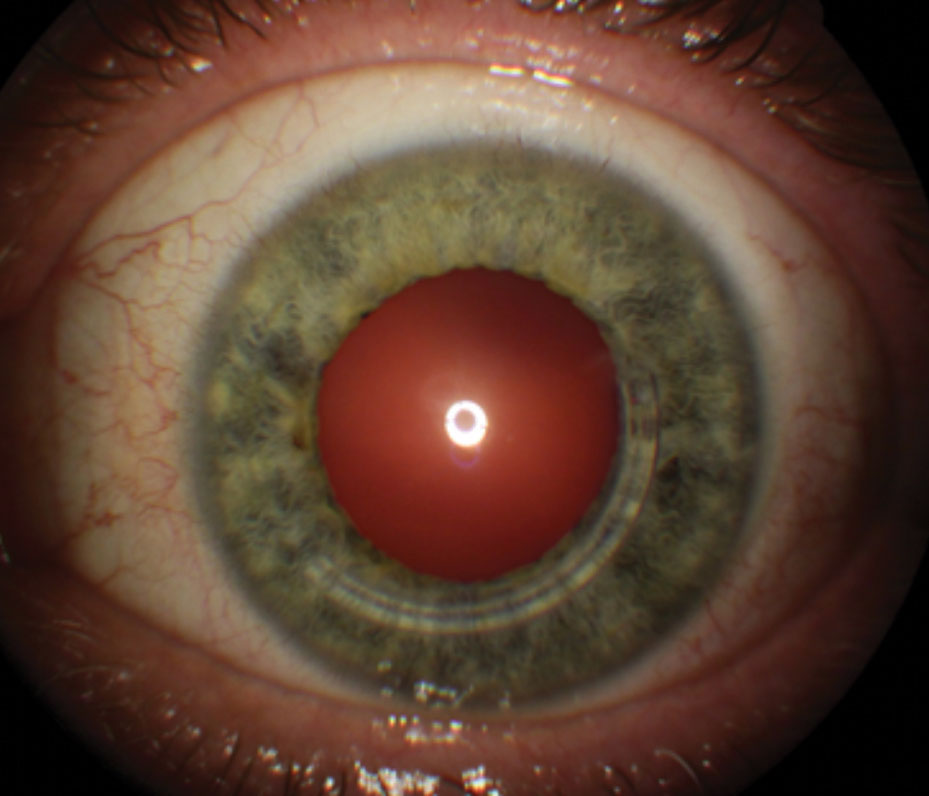 |
| Fig. 3. Intacs for keratoconus, showing inferotemporal placement of a single segment. |
Incremental Improvement
During the ’80s and ’90s, the efforts to improve treatment were largely unsuccessful. Epikeratoplasty gained brief surgical traction, as it frequently improved uncorrected and best-spectacle corrected visual acuity.8 But it fell out of favor quickly because the visual outcome of PK was generally preferable.8,9 The now-defunct SoftPerm hybrid lens sought to combine the benefit of RGP optics with soft lens comfort and stability. However, problems were common with this lens design, including junctional separation in 29.1% of cases, peripheral corneal neovascularization in 25% and discomfort in 29.1%.10 Other contact lens efforts included thick soft lens designs, such as the Flexlens Tri-curve keratoconus (X-Cel Contacts) to mask corneal irregularity and the Flexlens piggyback (X-Cel Contacts), which has a countersunk anterior cut-out to hold a corneal RGP lens. Two semi-scleral lenses that surfaced in the early 2000s were the Epicon (Specialty Ultra-Vision) 13.5mm diameter molded RGP lens and MacroLens (C&H Contact Lens), which came in sizes between 13.9mm and 15.0mm; both have since been discontinued.
In late 2011, Alden Optical (now part of Bausch + Lomb Specialty Vision Products) introduced the NovaKone toric lens, a thick soft lens made with hioxifilcon D designed to mask corneal irregularity.11 In 2012, B+L announced a global licensing agreement with UltraVision CLPL for KeraSoft IC, a lathed thick silicone hydrogel lens for irregular corneas, including those with keratoconus.12 All these lenses have a thickness of greater than 0.40mm, so even with a latheable silicone hydrogel material the resulting Dk/t does not meet the Holden-Mertz criteria of 24 x 10-9 units to avert hypoxia in daily wear. Clinically, the observed absence of corneal hypoxic findings in wearers of these thick lenses suggests that a significant amount of tear exchange occurs during lens wear.
The newer-generation hybrid contact lenses for keratoconus launched with SynergEyes KC in 2006, SynergEyes ClearKone in 2009 and the silicone hydrogel skirt hybrid lens, SynergEyes UltraHealth in 2013 (Figure 2). These lenses, unlike their SoftPerm predecessor, do not suffer from junctional separation and offer greater parameter customization and generally better outcomes. Their promise is to offer rigid quality vision with the centration, stability and comfort of a soft contact lens. As with corneal GP lenses, if hybrid lenses cause epitheliopathy and associated wearing discomfort, piggybacking may help.13
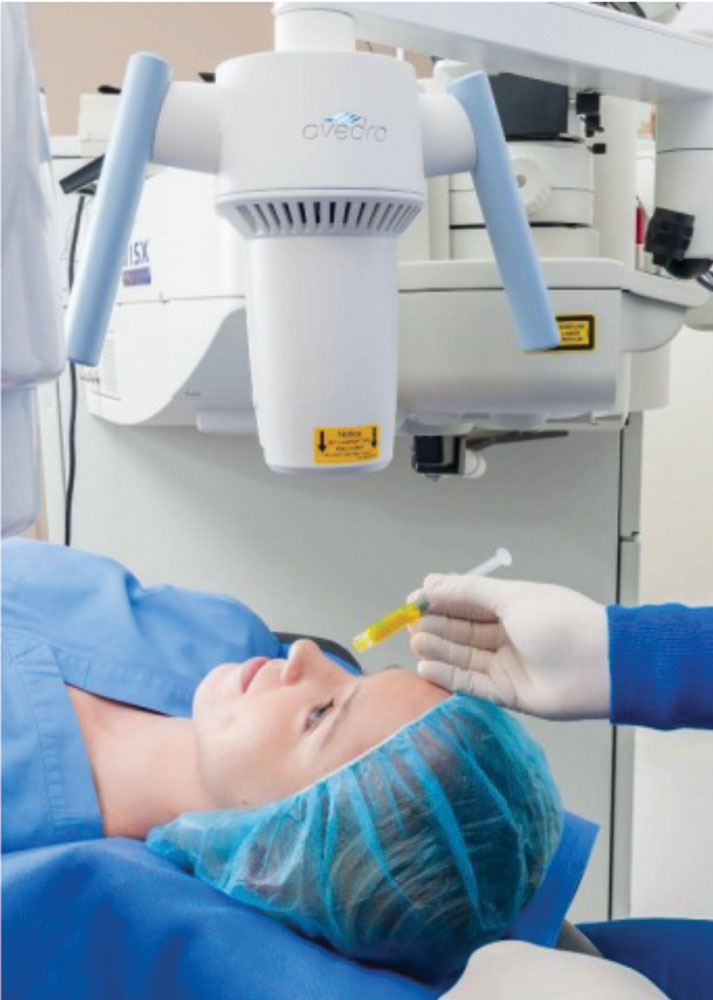 |
| Fig. 4. The Avedro KXL in action. Image by Avedro. |
Medical Management Gamechangers
Since the first Intacs surgery for keratoconus in 1997, reports continue to corroborate that it can improve unaided and best-spectacle corrected visual acuity and reduce corneal irregularity (Figure 3).14-16 Unlike excimer photoablative surgery, which subtracts from the corneal tissue in the visual axis, Intacs intracorneal ring segments bolster the mid-peripheral corneal thickness and indirectly reduce central corneal curvature. In 2004, Intacs received humanitarian device exemption FDA approval for treating keratoconus when corneal transplantation is the only remaining option to improve a patient’s functional vision.17
Just a year before Intacs’s FDA approval, researchers introduced corneal crosslinking (CXL) for keratoconus. Since then, the procedure has garnered significant interest due to its technical simplicity and ability to slow or halt progression while reducing corneal irregularity. Avedro received FDA approval for its crosslinking system (called KXL) in 2016; the modality is increasingly accepted as a standard treatment for patients with progressive keratoconus (Figure 4).18
Now that this treatment is available, detecting keratoconus before significant clinical progression is crucial to minimize loss of best-spectacle corrected acuity, reduce risk of corneal scarring and the need for PK. Since the disease generally self-arrests by the third to fourth decade of life, no consensus exists yet on whether CXL has a role for older keratoconus patients.19
As the understanding of CXL matures, clinicians must come to an agreement on what defines progression. Characteristics of progression include steepening of the anterior and posterior cornea surfaces, thinning of the cornea and worsening best-spectacle corrected visual acuity, although it remains debatable how much change in these indicators qualifies as progression. Furthermore, progression can be difficult to determine in patients who are wearing corneal RGP contact lenses due to corneal molding and spectacle blur. A true determination in these cases would require a complete RGP wash-out and monitoring for several months, if not longer, which is generally impractical.
 |
Fig. 5. Scleral lens showing optimal landing onto the bulbar conjunctiva while vaulting over the corneal surface. Image by Vision Research institute at Michigan College of Optometry. Click image to enlarge. |
Epi-on vs. Epi-off
The Avedro KXL system is indicated for treatment of progressive keratoconus in patients ages 14 and older by first debriding the corneal epithelium.18 While the “Dresden protocol” with epithelial removal is an established standard, some corneal surgeons are now performing variations of CXL, including trans-epithelial (epi-on) application of riboflavin drops. The proposed benefits of epi-on CXL are faster recovery and less postoperative discomfort. There is debate over how well riboflavin can permeate into the corneal stroma without epithelial debridement.
Increased CoverageIn the wake of Avedro’s KXL system approval and industry-wide adoption, 62 insurance carriers now cover the procedure, up from just three at the beginning of 2017.1 However, medical insurance coverage for CXL is only for the standard FDA-approved epi-off Dresden protocol. In addition, private-paying patients undergoing CXL are facing an increased cost as of July 1, 2017, when Avedro raised pricing for its system’s two photosensitizing agents, Photrexa and Photrexa Viscous, from $595 to $2,850 per eye.2 This was to reflect a more sustainable pricing, according to the company.2
|
So far, studies show similar outcomes between epi-on and epi-off CXL.20 In a 2018 meta-analysis, researchers found that, “analysis of the limited number of comparative studies available seems to demonstrate that standard CXL and trans-epithelial CXL have a comparable effect on visual, refractive, pachymetric and endothelial parameters at one year.”20 A double-masked clinical trial sponsored by Avedro commenced in 2017 with the intent of submitting data to the FDA on the epi-on procedure.21,22 Trans-epithelial CXL may fall within optometric scope of care for certain states with regulatory approval and industry acceptance of safety and efficacy.
Other variations of trans-epithelial CXL are under investigation as well, including the addition of iontophoresis, which uses a voltage gradient across the cornea to draw riboflavin into the stroma. One study found significant visual and refractive improvements 12 months after trans-epithelial CXL with iontophoresis, though the average improvement in corneal topography readings was slightly lower than the Dresden protocol in the same period.23 Despite the faster clinical recovery of visual performance with iontophoresis, the authors still recommend standard CXL for patients younger than 24 years of age. However, one case series found a high rate of early postoperative epithelial complications undermining the purported advantages of trans-epithelial CXL.24 Other variants of the Dresden protocol include performing CXL in combination with Intacs and photorefractive keratectomy. While the Dresden protocol is the current standard for CXL, it is likely that the favored CXL procedure will evolve.
 |
| Fig. 6. This is 3D image of the impression of a patient’s eye with a filtration bleb. Image by EyePrint Prosthetics. |
Contact Lens Game-changers
Notwithstanding the many advances for keratoconus, no therapy so far can replicate the smooth refracting surface and visual quality as well as a rigid contact lens. For the vast majority with keratoconus—even those who have undergone CXL and Intacs—rigid contact lens optics are still necessary for optimal vision. In this area, scleral lenses have revolutionized contact lens treatment for keratoconus.
Because scleral lenses vault over the sensitive corneal surface and land on the relatively insensitive bulbar conjunctiva overlying the sclera, they generally provide exceptional wearing comfort (Figure 5). They trap non-preserved saline against the ocular surface and are thus an effective treatment for severe dry eye.25 Research shows scleral lenses reduce biomarkers of dry eye in keratoconus.26
One study found that most keratoconus patients strongly prefer scleral lenses over traditional corneal RGP and hybrid contact lenses for vision and wearing comfort.27 Unlike corneal RGP lenses, scleral lenses are stable on the eye in various fields of gaze, are not subject to dislodging or decentering and are not susceptible to exogenous debris under the lens once seated on the eye. Because scleral lenses vault the cornea, they do not subject the patient to spectacle blur and visual drift after lens removal. In addition, the ability to specify front-surface toricity with scleral lenses can address residual astigmatism better than corneal RGPs due to the greater stability of scleral lenses.
Some scleral lens designs allow for asymmetrical lens specifications to pattern the ocular shape. For example, BostonSight’s scleral allows for a quadrant-specific toric haptic system, and the Zenlens design (Bausch + Lomb) can incorporate microvaults to avoid compressing conjunctival obstacles such as pingueculae.
Be Careful with BillingSince prescribing contact lenses for keratoconus requires greater expertise and chair time, these services command higher fees and reimbursements from third-party payers with provisions for medically necessary contact lenses. This also means these third-party payers are increasingly scrutinizing payments for this category by way of friendly “quality” audits and more ominous “targeted” audits. Providers must be careful to adhere to proper billing and coding practices as described by their provider manuals and agreements. For example, some vision plans with necessary contact lens authorizations will reimburse for keratoconus regardless of severity of the disease. Other plans require the keratoconus to meet certain severity criteria before qualifying for reimbursement. Some ambiguity exists with billing and coding for major vision plans. For example, EyeMed Vision Care currently reimburses contact lens services and materials for keratoconus at a higher level if the patient has “moderate/advanced” disease. EyeMed’s present claim form connects moderate/advanced disease with the ICD-10 classification for “unstable” disease, even though severity and stability are independent characteristics. For example, a 70-year-old keratoconus patient can have advanced disease (e.g., exhibit Munson’s sign) despite having stable ectasia. Conversely, a 15-year-old with mild or forme fruste keratoconus topographical pattern may have progressing, unstable keratoconus. Due to the inability of the ICD-10 codes to indicate severity of keratoconus, EyeMed has updated its provider manual to clarify that qualification for “moderate/severe” disease status is based on the presence of at least one of the following: corneal scarring, topographical steep K of 53D or higher, corneal thickness less than 476µm, or an unmeasurable refraction. In situations of ambiguity, always consult the vision plan’s representatives and provider manual for guidance. In an interview on this topic, Denis Humphreys, OD, VSP’s director of optometry, said, “While VSP supports medically necessary contact lenses (NCL) for ‘unstable,’ ‘stable’ and ‘unspecified’ keratoconus, we don’t pay doctors a higher amount based on the severity of the disease (i.e., a specific diagnosis code). Our NCL maximum payable amounts do not vary by diagnosis code and are inclusive of chair time required for lens fitting and evaluation.” Dr. Humphreys added, “VSP billing for NCL always requires an appropriate diagnosis that supports clinical findings and reflects a suitable type of contact lens fit. Our billing and reimbursement procedures are detailed in the Contact Lenses section of the VSP Provider Reference Manual, which is available online to all member doctors.” Other aspects of our updated understanding of keratoconus have not permeated into the administrative systems for rendering care. The ICD-10 classification system still allows the provider to code for unilateral keratoconus, even though the global consensus is that there is no such thing.3 Additionally, since PMD and keratoconus are believed to represent different presentations of the same disease, most cases of “crab-claw” topographies should be coded using the ICD-10 code for keratoconus and not for peripheral corneal degeneration.3 |
An impression-based prosthetic cover shell, EyePrintPro (EyePrint Prosthetics), can also provide scleral lens benefits for patients with keratoconus and may be particularly helpful for those with elevated conjunctival lesions, such as filtration blebs and pingueculae (Figure 6). According to Christine Sindt, OD, President of EyePrint Prosthetics, the company’s shell can “more closely predict and align the visual axis with a more even tear layer over the cornea. The true rotational stability of the device enables the use of premium optics, like multifocal and prism, in any direction, not just as a ballast. It also allows for more accurate predictions of clearances.”
The present-day renaissance for managing keratoconus is highlighted by several viable treatments—including CXL and scleral lenses—that compellingly improve the lives of many of these patients. Resources such as the National Keratoconus Foundation (www.nkcf.org) and Scleral Lens Education Society (www.sclerallens.org) offer valuable information for both patients and practitioners. Optometrists committed to this specialty can forget the frustrations of the past and enjoy the professional fulfillment of helping these patients while gaining shelter from the market forces disrupting mainstream optometry.
Dr. Chou practices in San Diego at ReVision Optometry, where he directs a referral-based keratoconus clinic. He also provides industry-supported workshops for eye care practitioners on prescribing scleral contact lenses. Dr. Chou reported the first case of Intacs surgery for keratoconus in the US during his fellowship in 1999 at Jules Stein Eye Institute, UCLA School of Medicine, and served as a clinical investigator for the SynergEyes KC lens prior to its FDA approval.
1. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthal. 1986;101(3):267-73. 2. Godefrooij DA, de Wit GA, Uiterwaal CS, et al. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169-72. 3. Gomes JA, Tan D, Rapuano CJ, et al. Global consensus of keratoconus and ectatic diseases. Cornea. 2015;34(4):359-69. 4. Koc M, Tekin K, Inanc M, et al. Crab claw pattern on corneal topography: pellucid marginal degeneration or inferior keratoconus? Eye. 2018;32:11-18. 5. Arnalich-Montiel F, Alio del Barrio JL, Alio JL. Corneal surgery in keratoconus: which type, which technique, which outcomes? Eye Vis (Lond). 2016;3:2. 6. Castroviejo R. Keratoplasty for the treatment of keratoconus. Trans Am Ophthalmol Soc. 1945;26:127-53. 7. Zadnik K, Barr JT, Edrington TB. Baseline findings in the Collaborative Longitudinal Evaluation of Keratonus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998:39:2537-46. 8. Waller SG, Steiner RF, Wagoner MD. Long-term results of epikeratoplasty for keratoconus. Cornea. 1995;14(1):84-8. 9. Wagoner MD, Smith SD, Rademaker WJ, et al. Penetrating keratoplasty vs epikeratoplasty for the surgical treatment of keratoconus. J Refract Surg. 2001;17(2):138-46. 10. Özkurt Y, Oral Y, Karaman A, et al. A retrospective case series: use of SoftPerm contact lenses in patients with keratoconus. Eye Contact Lens. 2007;33(2):103-5. 11. Alden Optical. NovaKone: a soft contact lens for keratoconus. Bausch + Lomb Specialty Vision Products. aldenoptical.com/products/soft-specialty/novakone/novakone. Accessed March 1, 2019. 12. Bausch + Lomb announces availability of KeraSoft IC contact lenses for keratoconus and other irregular corneas. Eyewire. January 1, 2012. eyewiretoday.com/2012/01/09/20120109-bausch__lomb_announces_availability_of_kerasoft_ic_contact_lenses_for_keratoconus_and_other_irregular_corneas. Accessed March 1, 2019. 13. Scheid T, Kaplan E. A Novel keratoconic piggyback fitting utilizing a SiH lens and a Synergeyes KC hybrid. siliconehydrogels.org/in_the_practice/mar_08.asp. March 2008. Accessed March 1, 2019. 14. Colin J. Intacs may be useful for select keratoconus correction. Ocular Surgery News. April 15, 1999. 15. Chou B, Boxer Wachler BS. Intacs for a keratocone: A promising new option? Review of Optometry. 2000;137(5):97-98. 16. Boxer Wahler BS, Chandra NS, Chou B, et al. Intacs for keratoconus. Ophthalmology. 2003;110(5):1031-40. 17. U.S. Food and Drug Administration. Humanitarian Device Exemption (HDE). Intacs prescription inserts for keratoconus. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=H040002. Accessed March 1, 2019. 18. Avedro. Avedro receives FDA approval for Photrexa Viscous, Photrexa and the KXL system for corneal cross-linking. investors.avedro.com/news-releases/news-release-details/avedro-receives-fda-approval-photrexar-viscous-photrexar-and. April 18, 2016. Accessed March 1, 2019. 19. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319. 20. Daizong W, Benhao S, Qi L, et al. Comparison of epithelium-off versus transepithelial corneal collagen cross-linking for keratoconus: a systematic review and meta-analysis. Cornea. 2018;37(8):1018-24. 21. Avedro. Avedro announces enrollment of first patient in u.s. pivotal phase 3 epi-on corneal cross-linking trial for progressive keratoconus. investors.avedro.com/news-releases/news-release-details/avedro-announces-enrollment-first-patient-us-pivotal-phase-3-epi. June 18, 2018. Accessed March 1, 2019. 22. Study to evaluate the safety and efficacy of epi-on corneal cross-linking in eyes with progressive keratoconus. www.clinicaltrials.gov/ct2/show/NCT03442751. Accessed March 1, 2019. 23. Lombardo M, Giannini D, Lombardo G, Serrao S. Randomized controlled trial comparing transepithelial corneal cross-linking using iontophoresis with the Dresden Protocol in progressive keratoconus. Ophthalmology. 2017;124(6):804-12. 24. Chow SSW, Chan TCY, Wong IYH, et al. Early epithelial complications of accelerated trans-epithelial corneal crosslinking in treatment of keratoconus: a case series. International Ophthalmology. 2018;38(6):2635-38. 25. Bavinger JC, DeLoss K, Mian SI. Scleral lens use in dry eye syndrome. Curr Opin Ophthalmol. 2015;26(4):319-24. 26. Carracedo G, Blanco MS, Martin-Gil A, et al. Short-term effect of scleral lens on the dry eye biomarkers in keratoconus. Optom Vis Sci. 2016;93(2):150-7. 27. Bergmanson JP, Walker MK, Johnson LA. Assessing scleral contact lens satisfaction in a keratoconus population. Optom Vis Sci. 2016;93(8):855-60. |

