 |
A 19-year-old Caucasian male presented with symptoms of progressive, deteriorating vision and a red painful left eye for six days. He had been seen at a local Urgent Care facility, where he was diagnosed with ocular allergies, and then by a local eye care provider who referred the patient to our tertiary care center.
The patient had a medical history of seizures and was developmentally delayed secondary to neonatal herpes simplex virus type 2 (HSV-2) encephalitis. Review of systems revealed symptoms consistent with an upper respiratory infection for seven days. All other medical, ocular and family histories were unremarkable, and the patient was otherwise systemically healthy.
Upon exam, unaided visual acuity was 20/400 in both eyes with pinhole improvement to 20/40 OD and 20/80 OS. There was relative afferent pupillary defect in the left eye; extraocular motility, confrontation visual fields and intraocular pressures were all otherwise unremarkable.
The slit lamp exam showed bilateral palpebral conjunctival follicles but no palpable preauricular lymph nodes. The ocular exam of the right eye was otherwise unremarkable. The left eye had significant diffuse episcleral injection with inferior corneal stellate keratic precipitates (Figure 1). There were 2+ anterior chamber cell and flare (SUN classification) and moderate vitreous cell mildly obscuring the view of the fundus.
The exam did uncover grade 3 optic disc edema (using the modified Frisén scale). The retinal vasculature was tortuous, and focal retinal whitening and hemorrhaging was seen in the superonasal periphery. (Figures 2 and 3).
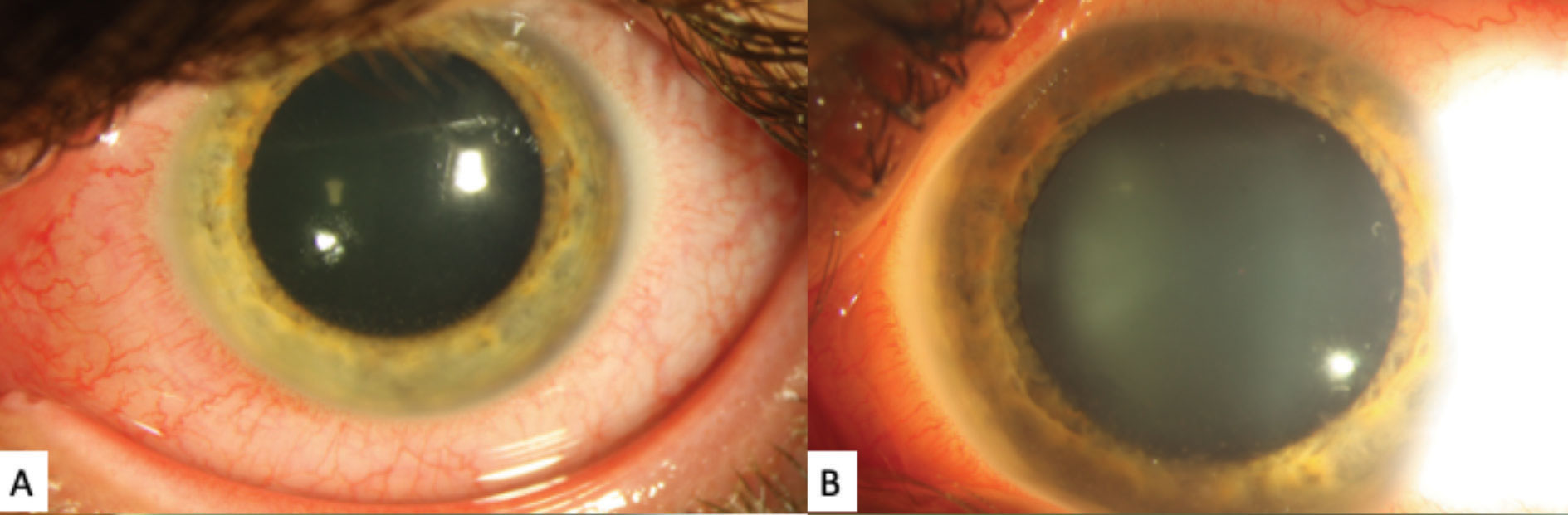 |
| Fig. 1. These gross external slit lamp photos of the patient’s left eye shows significant diffuse episcleral injection. A faint posterior corneal opacities can be see, although the detail is poor. Click image to enlarge. |
Differential Diagnosis
A number of disease entities could underly our patient’s presentation. Conditions that can present with panuveitis could include sarcoidosis, tuberculosis, syphilis, Behçet disease, Vogt-Koyanagi-Harada syndrome and toxoplasmosis. One must also consider the viral entities that cause a necrotizing retinitis, such as varicella zoster virus (VZV), herpes simplex virus type 1 or 2 (HSV-1 or HSV-2), cytomegalovirus (CMV) and Epstein-Barr virus (EBV).
Analysis of blood serum can evaluate for syphilis, tuberculosis and toxoplasmosis. Aqueous and vitreous humor can be analyzed by polymerase chain reaction (PCR) to detect for the presence of VZV, HSV-1, HSV-2, CMV and EBV. When other diagnostic modalities have failed, send vitreous humor to pathology for histological evaluation. Computed tomography (CT) of the chest is essential to evaluate for sarcoidosis and tuberculosis.
Diagnosis
We saw reason to highly suspect acute retinal necrosis due to the clinical presentation and known history of neonatal HSV-2 encephalitis. A diagnostic anterior chamber paracentesis was performed on the left eye, and the aqueous humor was sent for PCR analysis for VZV, HSV-1, HSV-2 and CMV; only HSV-2 was detected. Serological studies revealed normal blood composition and were negative for syphilis and toxoplasma titers. A negative CT ruled out sarcoidosis and tuberculosis.
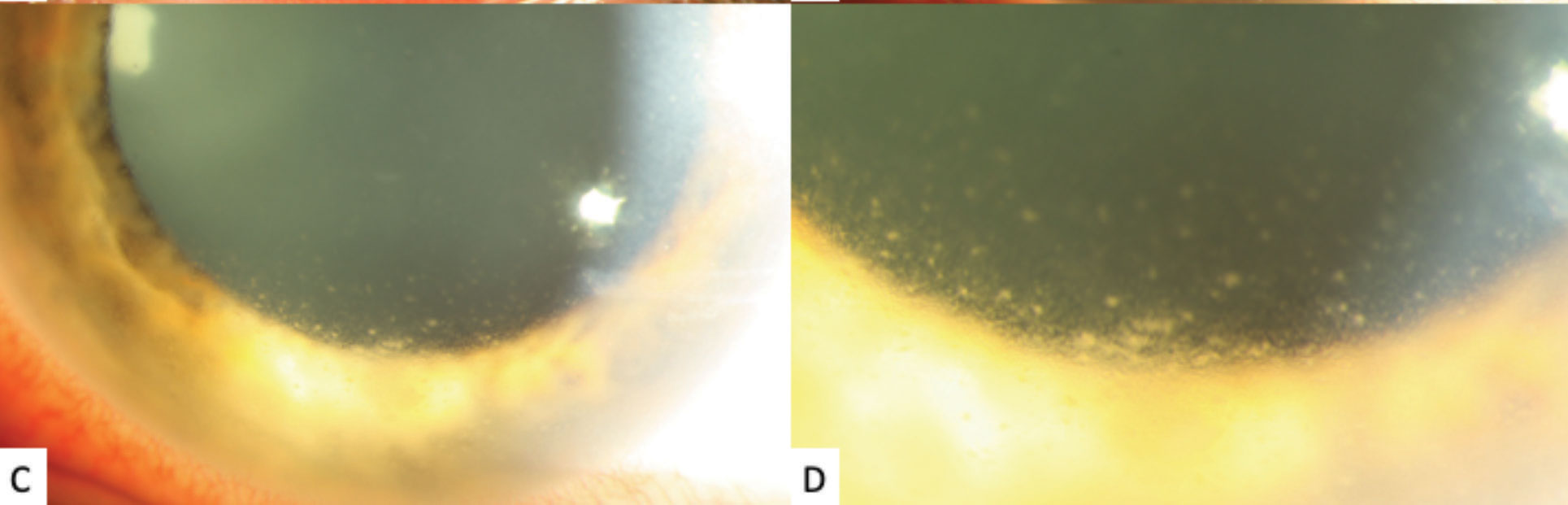 |
| Fig. 1c and 1d. High-magnification slit lamp photographs of the left eye showing inferior stellate keratic precipitates on the lower half of the cornea. Click image to enlarge. |
Discussion
While rare, acute retinal necrosis (ARN) is a potentially visually devastating condition of immunocompetent patients.1,2 Although initially unilateral, the fellow eye can be involved within three to four weeks if untreated but can also occur decades after the initial presentation.3,4
Infectious etiology is due to the Herpesviridae family, most commonly by VZV, followed by HSV-1 and HSV-2; average age at onset is 52.4 years for VZV, 44.3 years for HSV-1, and 24.3 years for HSV-2.5,6 Researchers have published no incidence data for ARN in the United States, but studies from the United Kingdom show a minimum annual incidence of 0.63 cases per million with a slight male predilection.7,8 Researchers suggested ARN has outbreak seasonality, with higher incidence in the first half of the year (winter and spring) and peak incidence in February.9 Prompt recognition and treatment is absolutely critical to optimize the visual prognosis in these patients.
ARN diagnosis is largely clinical. The American Uveitis Society (AUS) defines the diagnostic criteria as acute panuveitis with focal or multifocal peripheral retinal necrosis, occlusive retinal vasculitis (predominantly arterioles) and rapid (circumferential) disease progression in the absence of antiviral therapy.1,3 Supporting clinical findings may include optic nerve involvement, scleritis and pain.1 Diagnostic aqueous or vitreous humor PCR can confirm ARN and isolate an etiological organism; samples from aqueous or vitreous humor are thought to be equivocal, but some studies suggest vitreous humor may have greater yield.8,10-13
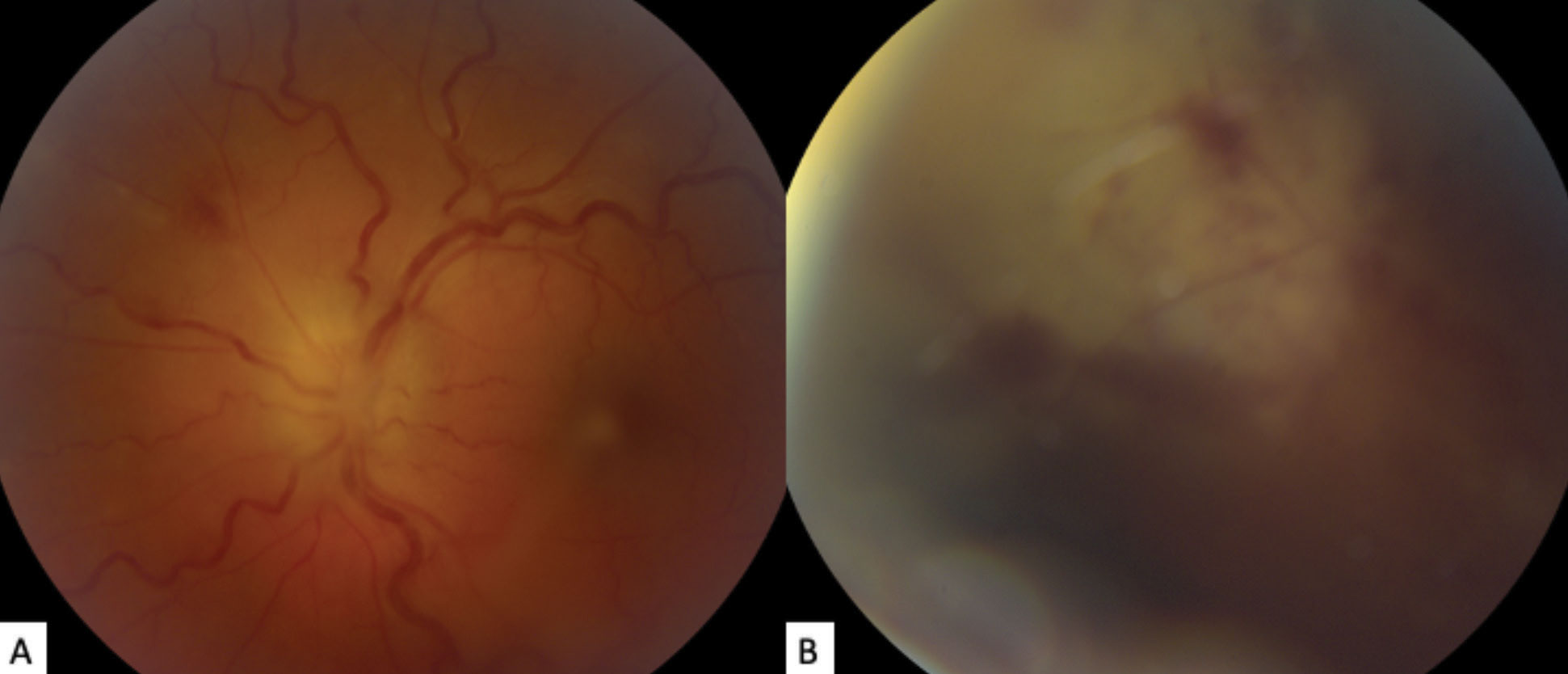 |
| Fig. 2. (a) The patient’s fundus photo shows their left eye’s grade 3 optic disc edema, using the modified Frisén scale. Vascular tortuosity, faint retinal whitening and hemorrhaging is also visible superonasally. (b) This photo of the superonasal peripheral fundus shows focal retinal whitening with hemorrhage obscured by vitreous haze. Click image to enlarge. |
Therapeutics
Goals of therapy are to halt progression in the affected eye and prevent involvement of the fellow eye.3,14-16 The mainstay of treatment involves systemic antiviral therapy with intravenous (IV) acyclovir for five to 10 days, followed by transition to an oral antiviral agent for six to eight weeks.3,4,10,12,14-17 Oral antiviral options include acyclovir, valacyclovir, famciclovir and valganciclovir; except for acyclovir, all are pro-drugs.
Of the oral antiviral agents, valacyclovir has the greatest bioavailability and penetration of acyclovir into the vitreous cavity.10,15,18 Moreover, high-dose oral valacyclovir (2g by mouth three times daily) can achieve vitreous concentrations comparable with IV acyclovir with a similar side effect profile.10,16,19
Moorfields Eye Hospital compared high-dose oral valacyclovir with IV acyclovir and demonstrated that they were clinically equivalent in best-corrected visual acuity, risk to developing a retinal detachment and safety.20 Oral valganciclovir can achieve concentrations comparable with IV ganciclovir, but it’s reserved mainly for CMV retinitis treatment.14 Intravitreal ganciclovir or foscarnet are generally second-line options for aggressive or refractory cases not responding to systemic therapy alone; however, combined intravitreal and systemic antiviral therapy may be better than systemic therapy alone (lower incidence of retinal detachment and severe visual loss of 20/200 or worse and higher incidence of better final visual acuity).10,14,17
Given the robust inflammatory response seen in the immunocompetent patients of ARN, corticosteroid therapy is often necessary to minimize damage to ocular structures.14,15 Oral administration is best and should be administered 24 to 48 hours after initiating systemic antiviral therapy.3,12,14 Platelet hyperaggregation has been observed in ARN and can be addressed with corticosteroids or anticoagulants such as aspirin.14
Complications of ARN can include retinal detachment, optic atrophy, vascular occlusion and involvement of the fellow eye.2,3,12 Research shows a high propensity for retinas to detach, both from the atrophic nature of the necrotic retina as well as secondary to a tractional component from downstream vitreous contraction.3,4,10
Some have favored prophylactic laser photocoagulation to strengthen chorioretinal adhesions as a barricade posterior to areas of retinitis.4,14 Although variable success has been reported, an obvious limitation to this option is vitreous inflammation and poor visibility impeding the ability to apply adequate laser.3,4,12 For this reason, less severe cases are likely to have a more favorable response and outcome; conversely more severe cases are the ones that are more prone to retinal detachment.3,4,12 Nevertheless, there is likely an indication to apply laser barricade as soon as the view allows.3,4
Although implementing early vitrectomy may reduce the rate of retinal detachment, one study showed final visual outcomes were equivalent with those who did not undergo early vitrectomy, likely owing to the multifactorial nature of vision loss in this population and significant role that optic atrophy can play.12
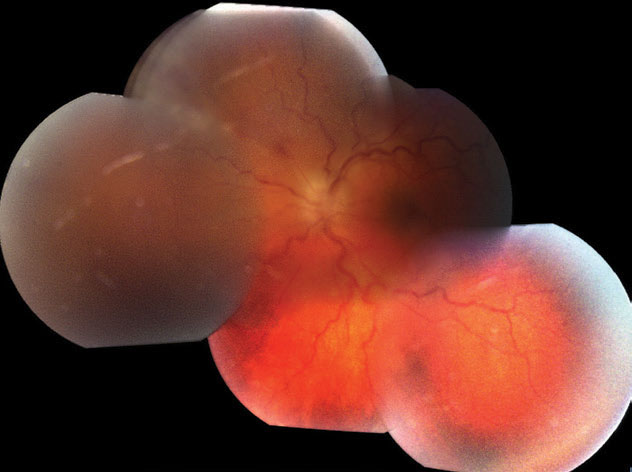 |
| Fig. 3. These montage fundus photos portray the patient’s mild vitreous haze, optic disc edema, vascular tortuosity and the posterior aspect of active retinitis superonasally. Click image to enlarge. |
Herpes Association
Multiple case reports have hypothesized that ARN can be a complication of either active or prior herpetic encephalitis.21,22 Given the rarity and mortality of herpetic encephalitis, very little data exists, so it is poorly described and understood.21,22
It would appear that anybody who has suffered herpetic encephalitis may be at an increased risk of herpetic eye disease given that the eye is an extension of the central nervous system. Moreover, it may be worthwhile to consider and discuss lifelong prophylaxis with these patients who have had herpetic encephalitis with or without ocular involvement, as well as those who have had unilateral ocular involvement in efforts to spare the fellow eye.21
Following Up
Our patient’s immediate management included in-patient admission for IV acyclovir with transition to oral valacyclovir, atropine eye drops and co-administration of prednisolone eye drops and oral prednisone after regression of retinitis was demonstrated at 36-hour follow-up. The patient received repeat intravitreal ganciclovir injections, prophylactic laser barricade, and was tapered off the steroids as the uveitis began to subside. Despite aggressive therapy, the patient was poorly compliant to maintenance dosing of oral valacyclovir and eventually reactivated with subsequent rhegmatogenous retinal detachment. Best-corrected acuity in the left eye was 20/250 at most recent follow-up (nine years since initial presentation).
Dr. Aboumourad practices at the Bascom Palmer Eye Institute in Miami.
| 1. Holland G. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117(5): 663-7. 2. Fisher J, Lewis M, Blumenkranz M, et al., The acute retinal necrosis syndrome. Part 1: Clinical manifestations. Ophthalmol. 1982;89(12):1309-16. 3. Culbertson W, Atherton S. Acute retinal necrosis and similar retinitis syndromes. Int Ophthalmol Clin. 1993;33(1):129-43. 4. Rodriguez-Garcia A, Foster C. Advances in the diagnosis and management of uveitis. Intechopen. May 29, 2019. 5. Gass JD. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, vol. 2. St. Louis: C.V. Mosby Company; 1987. 6. Yanoff M, Duker JS, eds. Ophthalmology. 5th ed. Philadelphia: Elsevier; 2019. 7. Muthiah M, Michaelides M, Child C, Mitchell S. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452-5. 8. Cochrane T, Silvestri G, McDowell C, et al. Acute retinal necrosis in the United Kingdom: results of a prospective surveillance study. Eye (Lond). 2012;26(3):370-8. 9. Hedayatfar A, Khorasani M, Behnia M, Sedaghat A. Seasonality of acute retinal necrosis. J Ophthalmic Vis Res. 2020;15(1):53-8. 10. Schoenberger S, Kim S, Thorne J, et al. Diagnosis and treatment of acute retinal necrosis: A report by the American Academy of Ophthalmology. Ophthalmol. 2017;124(3):382-92. 11. Fine H, Burke S, Albini T, Toxoplasmosis retinitis masquerading as acute retinal necrosis. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):895-9. 12. Hillenkamp J, Nölle B, Bruns C, et al. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmol. 2009;116(10):1971-5.e2. 13. Williams A, Nguyen V, Botsford B, Eller A. Bilateral acute retinal necrosis caused by two separate viral etiologies. Am J Ophthalmol Case Rep. 2020;18(2):100636. 14. Shantha J, Weissman H, Debiec M, et al., Advances in the management of acute retinal necrosis. Int Ophthalmol Clin, 2015;55(3):1-13. 15. Taylor S, Hamilton R, Hooper C, et al, Valacyclovir in the treatment of acute retinal necrosis. BMC Ophthalmol. 2012;12(9):48. 16. Liu T, et al., Valacyclovir as initial treatment for acute retinal necrosis: A pharmacokinetic modeling and aimulation study. Curr Eye Res. 2017;42(7):1035-8. 17. Luu K, Scott I, Chaudhry N, et al. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am J Ophthalmol. 2000;129(6):811-3. 18. Acosta E, Fletcher C, Valacyclovir. Ann Pharmacother. 1997;31(2):185-91. 19. Huynh T, Johnson M, Comer G, et al., Vitreous penetration of orally administered valacyclovir. Am J Ophthalmol. 2008;145(4):682-6. 20. Baltinas J, Lightman S, Tomkins-Netzer O. Comparing treatment of acute retinal necrosis with either oral valacyclovir or intravenous acyclovir. Am J Ophthalmol. 2018;188(4):173-80. 21. Kianersi F, Masjedi A, Ghanbari H. Acute retinal necrosis after herpetic encephalitis. Case Rep Ophthalmol. 2010;1(2):85-9. 22. Okafor K, Lu J, Thinda S, et al. Acute retinal necrosis presenting in developmentally-delayed patients with neonatal encephalitis: A case series and literature review. Ocul Immunol Inflamm. 2017;25(4):563-8. |

