26th Annual Surgery Report-Pearls of Postoperative Care |
As a bridge between topical or laser treatment and incisional glaucoma surgery, minimally invasive glaucoma surgery (MIGS)—of which there are many—is growing in popularity. These procedures aim to provide a safer and less invasive means of reducing intraocular pressure (IOP) than traditional incisional surgery, while also decreasing dependency on topical hypotensive medications. The magnitude of IOP lowering depends largely on the respective mechanism of action of MIGS.
Here, we discuss various MIGS approaches, broken down by anatomical area, and the pertinent patient selection criteria. The clinical information will help clinicians competently incorporate MIGS into their surgical referral repertoire and select the best MIGS according to individual patient needs.
Note: This article does not include a discussion of surgical modalities that aim to reduce aqueous humor production by cyclodestruction of the ciliary body, such as endocyclophotocoagulation and transscleral cyclophotocoagulation.
Trabecular Meshwork Bypass

|
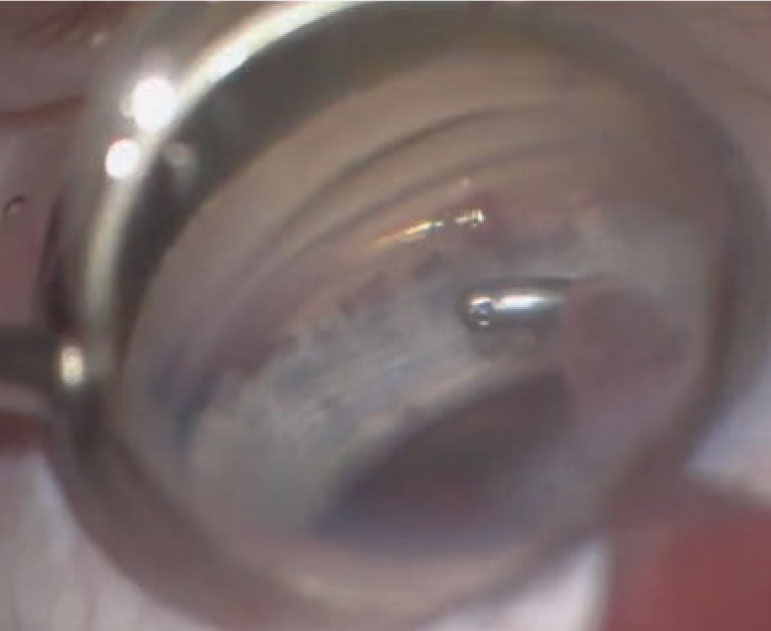
| iStent inject insertion directly following cataract surgery. (Image courtesy of Rachel Caywood, OD.) Click image to enlarge. |
The iStent (Glaukos) is used in combination with cataract surgery for patients with mild to moderate glaucoma. Ab interno placement of the L-shaped device improves the aqueous outflow by creating a channel through the trabecular meshwork (TM).
After two years, research shows no statistically significant difference in IOP between the iStent with phacoemulsification group (8.4mm Hg lower than baseline) and the cataract surgery alone group (7.5mm Hg lower).1 At the 24-month follow-up, 61% of the combination group maintained an IOP ≤21mm Hg without medication compared with only 50% of the control group.1
Although efficacy was modest, the iStent achieved a significant prolonged reduction in IOP as well as a reduction in topical medication burden. The safety profile is favorable and comparable with cataract surgery alone, the most common complications being transient IOP elevation and transient hyphema (commonly self-limiting).
Adverse events of stent obstruction, blockage or malposition, although they often only require monitoring, can be addressed with laser or surgical intervention.
The iStent Inject (Glaukos), a second-generation model approved in 2018, is also used in combination with cataract surgery for patients with mild to moderate glaucoma. It was developed with the premise that multiple iStents may be more effective than a single iStent. The implant is smaller in size and contains four inlets vs. one, allowing a multidirectional outflow of aqueous.2
A multicenter clinical trial comparing cataract surgery and iStent Inject with cataract surgery alone found that 75.8% of patients in the treatment group achieved a ≥20% reduction in medication-free IOP from baseline at 24 months compared with 61.9% in the control group. The mean reduction in medication-free IOP from baseline to 24 months was modestly improved with the combination group: 7.0 ± 4.0mm Hg vs. 5.4 ± 3.7mm Hg.3
 |
| Once the Hydrus device is in place, the surgeon confirms good stent placement before completing the procedure. (Image courtesy of Brooke Mathie, OD.) Click image to enlarge. |
The Hydrus Microstent (Ivantis) was FDA-approved in 2018 for use in combination with cataract surgery for mild to moderate glaucoma. The microstent is threaded into Schlemm’s canal using an ab interno approach. After the implantation, the Hydrus dilates and expands the diameter of three clock hours of Schlemm’s canal. This allows for enhanced aqueous outflow by providing TM bypass and direct access to multiple collector channels.
The Hydrus II study found 80% of patients treated with Hydrus and phacoemulsification achieved a 20% reduction from baseline IOP at 24 months compared with 46% of patients in the phacoemulsification alone group. In addition, 72.9% in the combination cohort remained free of topical medications vs. 37.8% in the control arm.4 A larger randomized trial, the HORIZON study, found similar results.5
The Hydrus implant also has a favorable safety profile. Complications, while infrequent, were most notably transient hyphema and early IOP spike from baseline in less than 10% of patients thought to be attributed to retained viscoelastic material. Also, the Hydrus II study found few subjects developed focal peripheral anterior synechiae, which did not require any further intervention.4
The COMARE study is the first to directly assess the efficacy of two different MIGS devices as stand-alone treatments.6 The study randomized both phakic and pseudophakic patients to receive either the Hydrus Microstent or two iStent trabecular bypass devices. Results demonstrated an improved success rate of the Hydrus over the two iStents in eliminating the need for medication use, 46.6% vs. 24%.6 Among eyes without medication, the Hydrus achieved an IOP ≤18mm Hg in 30.1% vs. 9.3% in the iStent group.6 While both MIGS devices had similar safety profiles, the Hydrus resulted in a higher surgical success rate and efficacy compared with the two iStents.
Ab-interno trabeculectomy (Trabectome, Microsurgical Technology) is a surgical technique that facilitates aqueous outflow by thermal ablation and removal of 30° to 180° of the TM and the inner wall of Schlemm’s canal, thereby exposing collecting channels.7
Whether performed in a stand-alone manner or in combination with cataract surgery, research shows a significant and consistent decrease in IOP from baseline.8 The success rate was nearly 85% at five years and 56% at 7.5 years (using the common definition of success of final IOP ≤21mm Hg with a 20% decrease from baseline).8
In addition, the Global Trabectome Outcomes study showed that, at 90 months, the average IOP demonstrated a 29% reduction from a baseline of 23.0mm Hg to 16.5mm Hg, and the average number of glaucoma medications decreased by 38% from 2.6 to 1.6.9
Patient Selection PearlsMIGS procedures are changing the treatment strategies for patients with glaucoma, and optometrists must be prepared to make MIGS recommendations based on ocular findings and patient factors. The goal is to individualize the treatment approach by matching the surgical benefits with the disease stage. These three clinical pearls are paramount when educating patients on their options: Lens status. Most MIGS are employed at the time of cataract surgery in patients with coexisting glaucoma. If cataract surgery is not indicated, a goniotomy or trabeculotomy procedure, cyclophotocoagulation or Xen gel stent are treatment choices. Disease type. In conjunction with cataract surgery, most trabecular bypass MIGS have demonstrated good surgical benefits for mild to moderate open-angle glaucoma. Secondary glaucomas, such as pigmentary or pseudoexfoliation, may respond well to trabecular bypass procedures and goniotomy or trabeculotomy procedures. For angle-closure glaucoma, goniosynechiolysis and goniotomy can be very effective. Surgeon selection. Glaucoma surgeons’ proficiency and access to MIGS vary greatly. Comanagement requires communication with the surgeon regarding their surgical procedural preference and the availability of certain MIGS devices.1
|
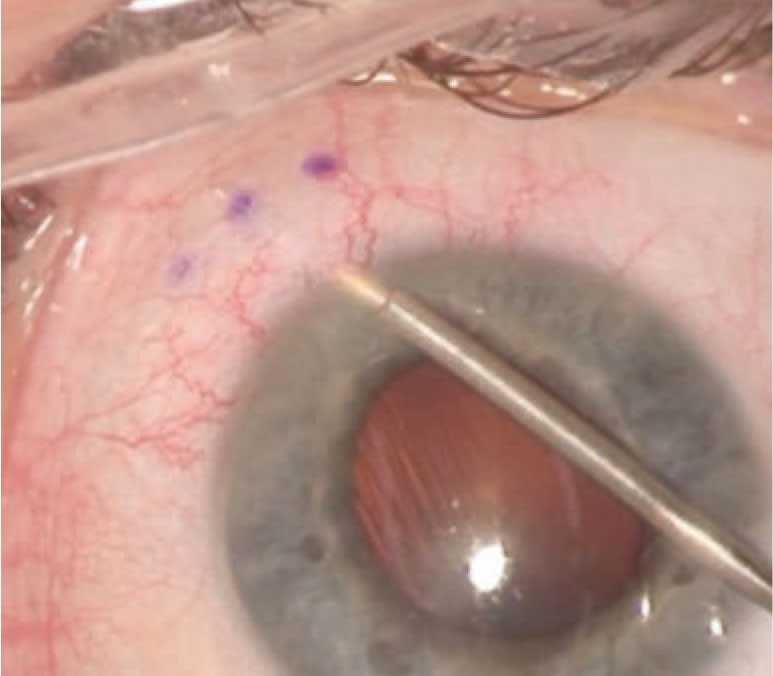 |
| The Xen gel stent creates a drainage pathway from the anterior chamber to the subconjunctival space to lower IOP. (Image courtesy of Justin Schweitzer, OD.) Click image to enlarge. |
The Kahook Dual Blade (KDB, New World Medical) is a surgical tool designed to remove a strip of TM tissue and the inner wall of Schlemm’s canal up to 180°.
Clinical studies demonstrate success as both a stand-alone and combined procedure with phacoemulsification. Both approaches can provide a significant and sustained reduction in IOP and medication burden. The six-month outcome of success, defined as IOP reduction of more than 20% from baseline and medical regimen reduced by more than one medication, was 69.8% and 67.9%, respectively.10 For the combined procedure, 12-month data indicated a 57.7% success rate for pressure reduction and 63.5% for treatment reduction.11,12
The efficacy is similar across disease severity, making this an effective and safe alternative to filtering surgery for patients with severe and refractory glaucoma.13 Goniotomy performed with KDB has an excellent safety profile, consistent with other ab-interno procedures, with rare complications of postoperative IOP spike and hyphema.
The Trab360 (Sight Sciences), released in 2015, provides an ab interno approach for access to 360° of Schlemm’s canal. The cannula tip of the device pierces the TM to gain access into Schlemm’s canal. A microcatheter is threaded through Schlemm’s canal, and the TM is inwardly opened by pulling on the ends of the catheter, either in one direction for 180° or in both directions for a total 360° trabeculotomy. It can be either stand-alone or in combination with cataract surgery.14
One study of refractory glaucoma patients who had stand-alone 360° trabeculotomy with Trab360 found 59% of eyes achieved ≥20% reduction in IOP and IOP <18mm Hg with the same or fewer medications at 12 months compared with baseline.15 Another study indicated success of Trab360 for treating primary congenital glaucoma.16 The Trab360 device demonstrates a favorable safety profile with mild transient hyphema as the most common adverse event noted.
Gonioscopy assisted transluminal trabeculotomy, an ab-interno circumferential trabeculotomy using a suture or microcatheter, is designed to decrease the proximal resistance of conventional outflow similarly to Trab360. One study showed patients with primary open-angle glaucoma demonstrated an IOP reduction of 7.7mm Hg at six months with an average of 0.9 fewer medications.17 Those with secondary glaucoma demonstrated an IOP reduction of 17.2mm Hg with an average of 2.2 fewer medications. These benefits were sustained in the 12-month study. Like other MIGS, the procedure has a favorable safety profile, with transient hyphema being the most common adverse event.
Schlemm’s Canal
Ab interno canaloplasty (ABiC, Ellex) is designed to catheterize and viscodilate Schlemm’s canal using the iTrack surgical system. The ABiC may be combined with cataract surgery or performed as a stand-alone procedure. The microcatheter is threaded into Schlemm’s canal for 360°. Upon withdrawal of the microcatheter, a viscoelastic solution is injected to enlarge the canal and create a circumferential flow.18 This results in reduction of outflow resistance through the TM, Schlemm’s canal and the distal outflow system, beginning with the collector channels.
Results of a retrospective case review of patients who had ABiC with or without cataract surgery showed that IOP fell from 20.4 ± 4.7mm Hg pre-op to 13.3 ± 1.9mm Hg 12 months post-op, and the medication burden decreased from 2.8 ± 0.9 to 1.1 ± 1.1.19 Furthermore, 40% of eyes post-procedure were medication-free.19
Subconjunctival Space
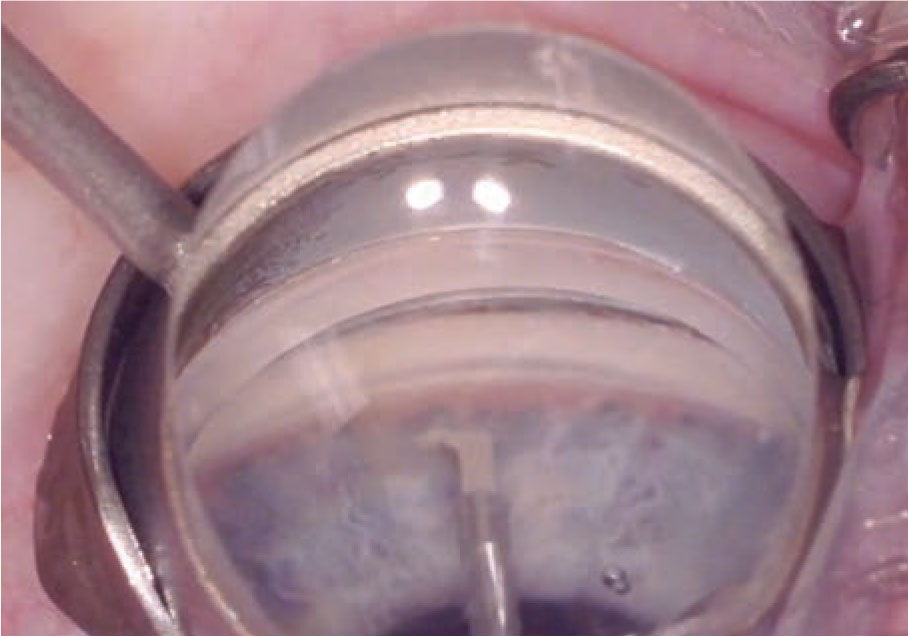 |
| The Kahook Dual Blade is designed to allow the surgeon to lift and excise the TM completely. (Image courtesy of Justin Schweitzer, OD.) Click image to enlarge. |
The Xen gel (Allergan) implant, FDA-approved in 2016, targets subconjunctival outflow with an ab interno approach. Made of biocompatible collagen-derived gelatin, the 6mm long tube with a lumen size of 45µm creates a drainage pathway between the anterior chamber and the subconjunctival space.
The Xen gel implantation may be performed as a stand-alone procedure or in combination with cataract surgery for patients with mild to moderate glaucoma. Several studies indicate a high success rate of lowering IOP to ≤18mm Hg, and high complete success, defined as pressure reduction ≤18mm Hg without any topical medication, all at one year post-op.20-22 The Xen gel implant can be efficacious in refractory glaucoma as well.23
Adverse events include transient anterior chamber bleed, transient hypotony, transient choroidal detachment and, more commonly, the need for additional needling. There is potentially a greater degree of postoperative management with Xen gel.
Currently, it is unclear if the efficacy, simplicity and safety profile of Xen gel outweigh the established efficacy of traditional filtering surgeries. The higher cost of Xen gel compared with standard filtering surgery is also a limiting factor.24
Suprachoroidal SpaceThe CyPass Micro-Stent (Alcon) was the first FDA-approved MIGS to target the suprachoroidal space, and early evidence showed significant IOP and medication reduction when combined with cataract extraction. However, the CyPass device underwent an FDA recall in August 2018 following a five-year data review from the COMPASS-XT study suggesting a clinically significant increase in corneal endothelial cell loss (3% risk of loss per year compared with 1% per year in controls).1 The American Society of Cataract and Refractive Surgery task force recommends clinicians monitor these patients with specular microscopy for the development of visually significant complications from endothelial cell loss. Implantation depth and retention rings visible with gonioscopy have an apparent correlation with the rate of endothelial cell loss; however, repositioning or removing the device is discouraged. A surgeon may attempt to clip the retention rings on the proximal end of the device, if necessary, to reduce protrusion into the anterior chamber.2
|
Final Thoughts
Each MIGS procedure offers its own benefits and limitations. These devices are relatively new, and the long-term safety, efficacy and reproducibility of the outcomes are still under investigation.
While topical medications have an overall favorable safety profile and moderate success in lowering IOP, the efficacy depends heavily on patient adherence. Non-compliance rates in glaucoma can vary from 24% to as high as 59%.25
MIGS procedures have the potential to adequately reduce IOP, decrease dependence on topical medications, provide an alternative to more invasive glaucoma surgeries and exhibit a favorable safety profile. These procedures are still trying to find their niche within glaucoma care, and ongoing research will help to improve clinicians’ understanding of the optimal selection.
Drs. Parikh and Cody work at the W.G. Hefner VA Medical Center in Salisbury, NC.
1. Craven ER, Katz LJ, Wells JM, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-45. 2. Pillunat LE, Erb C, Jünemann AGM, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583-600. 3. Samuelson W, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811-21. 4. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283-93. 5. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study. Ophthalmology. 2019;126(1):29-37. 6. Ahmed IK, Fea A, Au L, et al. A prospective randomized trial comparing Hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: The COMPARE study. Ophthalmology. 2020;127(1):52-61. 7. Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34(7):1096-103. 8. Kaplowitz K, Bussel I, Honkanen R, et al. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol. 2016;100(5):594-600. 9. Okeke CO, Miller-Ellis E, Rojas M, Trabectome Study Group. Trabectome success factors. Medicine. 2017;96(24):e7061. 10. Berdahl JP, Gallardo MJ, ElMallah MK, et al. Six-month outcomes of goniotomy performed with the Kahook Dual Blade as a stand-alone glaucoma procedure. Adv Ther. 2018;35(11):2093-102. 11. Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-month outcomes of goniotomy performed using the Kahook Dual Blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35(9):1460-69. 12. Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43(9):1197-1201. 13. Salinas L, Chaudhary A, Berdahl JP, et al. Goniotomy using the Kahook Dual Blade in severe and refractory glaucoma: 6-month outcomes. J Glaucoma. 2018;27(10):849-55. 14. Bettin P, Khaw PT, eds. Glaucoma Surgery. 2nd ed. Basel, Switzerland: Karger; 2017:127-46. 15. Sarkisian SR, Mathews B, Ding K, et al. 360° ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161-68. 16. Areaux RG Jr, Grajewski AL, Balasubramaniam S, et al. Trabeculotomy ab interno with the Trab360 device for childhood glaucomas. Am J Ophthalmol. 2020;209:178-86. 17. Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855-61. 18. Bettin P, Khaw PT, eds. Glaucoma Surgery. 2nd ed. Basel, Switzerland: Karger; 2017:130-46. 19. Gallardo M, Supnet R, Ahmed II. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol. 2018;12:2149-55. 20. Galal A, Bilgic A, Eltanamly R, et al. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246. 21. Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, et al. [Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions]. Arch Soc Esp Oftalmol. 2016;91(9):415-21. 22. De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Internat Ophthalmol. 2018;38(3):1129-34. 23. Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25-36. 24. Chaudhary A, Salinas L, Guidotti J, et al. XEN Gel Implant: a new surgical approach in glaucoma. Exp Rev Med Dev. 2018;15(1):47-59. 25. Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol. 2006;17(2):190-5. |

