24th Annual Surgery ReportFollow the links below to read other articles from annual update on surgery: The Preoperative Ocular Surface Checkup Understanding the Role of IOL Optics in Postoperative Vision Complaints Corneal Compromise: How to Assess the Risk of Post-LASIK Ectasia |
Minimally invasive glaucoma surgery (MIGS) has taken the market by storm as a new and exciting treatment for glaucoma. Yet, as with any new category, surgeons and comanaging optometrists are still learning about efficacy, mechanism of action and optimal use, especially for procedures involving stents placed in the trabecular meshwork. These uncertainties stem, in part, from the scarcity of research capable of fully mapping the anatomy of the limbus. For example, given the size of the stents currently on the market and the measured size of the canal of Schlemm, some stents may be too large to achieve increased aqueous outflow via the traditional pathway and may be instead more akin to the uveoscleral route of outflow.
Our research at the Texas Eye Research and Technology Center, focusing on mapping the limbus in three dimensions, hopes to provide a better understanding of the limbal anatomy to improve our understanding of this structure and how MIGS interacts with the local angle structures.
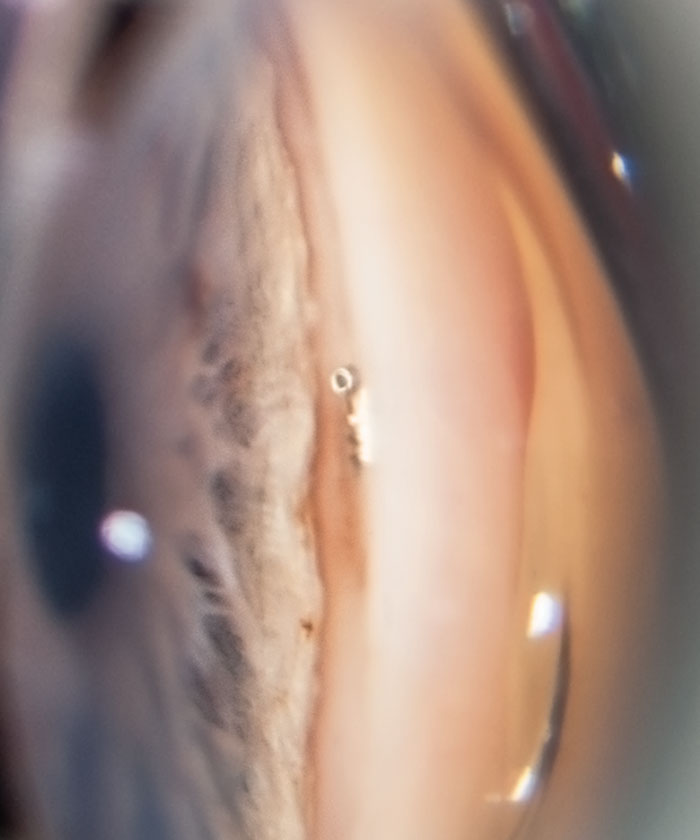 |
| Fig. 1. A post-op gonioscopic photo shows the iStent device implanted in the trabecular meshwork during the time of cataract surgery. The iStent requires no additional port incisions to be implanted. Photo by Anthony Van Alstine, OD, MS, and James M. Caruso, OD. |
The Problem at Hand
Glaucoma is the leading cause of irreversible blindness worldwide and a disease that optometrists frequently manage.1 It is a complex, multifactorial pathology involving structure as well as physiology, and the precise detailing of this disease, while generally understood, remains imperfect. The only known modifiable risk factor is elevated intraocular pressure (IOP), and the Ocular Hypertension Treatment Study shows that topical ocular hypotensive medications are effective in delaying the development of primary open-angle glaucoma.2
Although typical first-line therapy consists of topical medications, these come with certain ocular and systemic side effects, such as change in iris color, conjunctival hyperemia, punctate epithelial erosion, bradycardia, hypotension and impaired renal function, as well as concerns regarding patient compliance.3 More invasive surgical procedures are typically reserved for patients who can no longer be managed with topical medications. Surprisingly, even with hypotensive treatments, some patients have recalcitrant high IOP or significant glaucomatous progression regardless of seemingly low IOP. Thus, the search for new and improved treatment approaches to combat glaucoma continues, most recently focusing on MIGS.
A New Solution
Three categories of MIGS procedures exist: those involving (1) the trabecular meshwork and Schlemm’s canal, (2) the suprachoroidal space or (3) the subconjunctival space. Our research into the morphology of the limbal region and how the different structures relate to each other perhaps applies best to devices that bypass the trabecular meshwork, which in turn may impact the surrounding anatomy.
Research suggests the trabecular meshwork is the point of greatest resistance for aqueous outflow and pinpoints the internal endothelial wall of Schlemm’s canal, its basement membrane and the underlying juxtacanalicular connective tissue as the location in particular.4-6 Though there is still much to understand about the fluid dynamics of aqueous outflow and the other contributors of resistance to outflow, bypassing the trabecular meshwork is the primary intent of the MIGS procedures involving stents within this region of the limbal anatomy.
 |
MIGS Questions
As promising as these procedures seem to be, they come with several concerns that warrant further investigation:
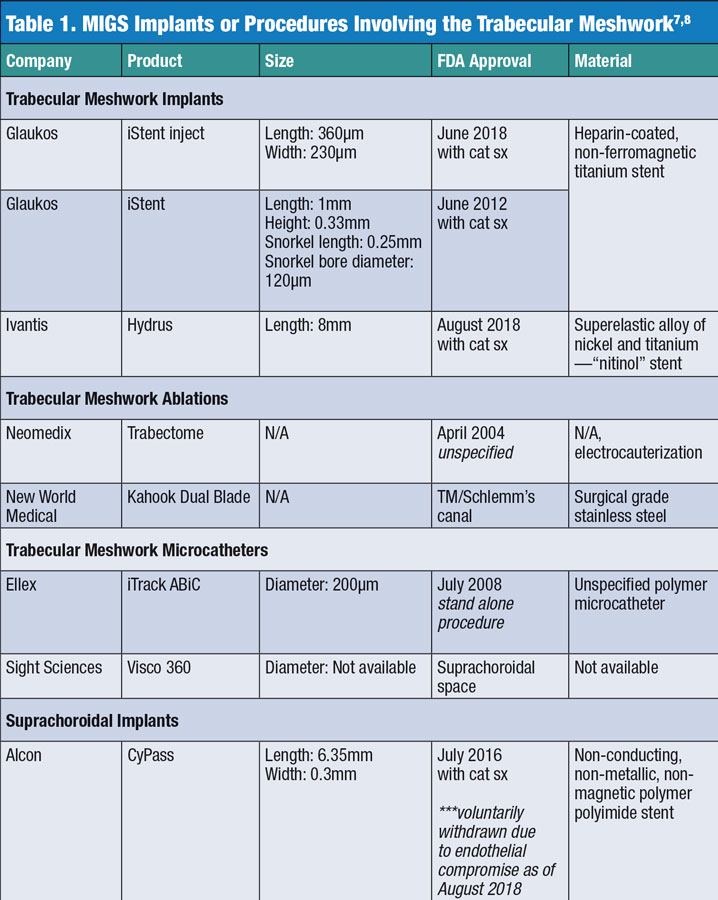
Efficacy. One challenge of studying these procedures is the FDA’s approval for use when they are implanted along with cataract surgery typical of devices such as the iStent (Glaukos), iStent inject (Glaukos) and CyPass (Alcon) (Table 1).7,8 This concurrent use can make it difficult to determine which of the two procedures has a more profound effect on IOP. Study data suggests MIGS are helpful but may not always be the major contributor to the lowering of IOP in a dual-procedure approach.
Additionally, studies that compare the decrease in IOP from cataract surgery alone have varied outcomes. One meta-analysis shows an enormous range within 22 studies, from 0.9% to 58.95% reduction, with the weighted mean decrease being 31%.9
Four studies involving the iStent’s concurrent placement during cataract surgery showed a statistically significant difference in IOP reduction when comparing the phacoemulsification-only group (4.7%), the phaco and iStent group (9%) and the phaco and two iStents group (27%).10 However, other studies within the meta-analysis suggest combined phaco and iStent provides a mean IOP reduction of 26%, not 9%.9 The meta-analysis also found the mean IOP reduction to be 26%, 18.4% and 20% when inserting one, two or three iStents along with phacoemulsification, respectively.9 These varying results make it difficult to estimate and predict the cumulative impact of the iStent placement. We must also consider the number of hypotensive agents used in this study—the stent(s) and the topical medications—making the independent hypotensive effect of the stents difficult to interpret without a washout period. In some cases, stent placement allowed a reduction in medication use more so than an additive IOP reduction if medication levels had been maintained.
Recently, studies of iStent placement as a stand-alone procedure have helped obtain a better understanding of its efficacy in the absence of phacoemulsification. In one study on pseudophakic eyes, a statistically significant reduction in IOP was noted with implantation of one iStent, although the mean number of postoperative hypotensive drugs used was not significantly different.11 Another study—with 117 out of 119 phakic eyes undergoing iStent implantation—reports a mean reduction of 7.6mm Hg (30%) IOP with one stent, an additional 1.6mm Hg (37% total) decrease with two stents and a 3.3mm Hg (43% total) decrease with three.12 The data indicates that the first stent appeared to provide the majority of the hypotensive effect, though the groups with multiple stents required fewer additions of topical hypotensive agents within the next 42 months.11 The mechanism for these trends remains to be understood, as the limbal anatomy and the associated fluid dynamics become clearer with continued research.
Medication reduction. Some practitioners posit that MIGS can potentially obviate the need for topical IOP-lowering drops altogether for post-op patients. In one meta-analysis, the maximum reduction in hypotensive medication use after phaco with iStent implantation occurred between 12 and 24 months, with a non-significant reduction at six months.9 The previously mentioned meta-analysis calculated the weighted mean reduction of topical glaucoma drugs after phaco alone at 1.01, 1.33 with the placement of iStent and phaco and 1.1 with two iStents and phaco.9 Curiously, the group with two iStents had a lesser medication reduction than the group with one iStent due to the effects of averaging the results in each cohort; thus, forecasting the treatment effect in any individual patient from these studies remains untenable.
The new Hydrus (Ivantis) implant shows promise, as it significantly decreases the need for topical medications even two years after the procedure.8 The FDA clinical trial for this device, the HORIZON study, shows a medication reduction of 0.4 at 24 months compared with controls.13 Additionally, an IOP reduction of equal to or greater than 20% was achieved in the stent group by 77.3% and in the non-stent group by 57.8%, which was found to be a statistically significant difference.13
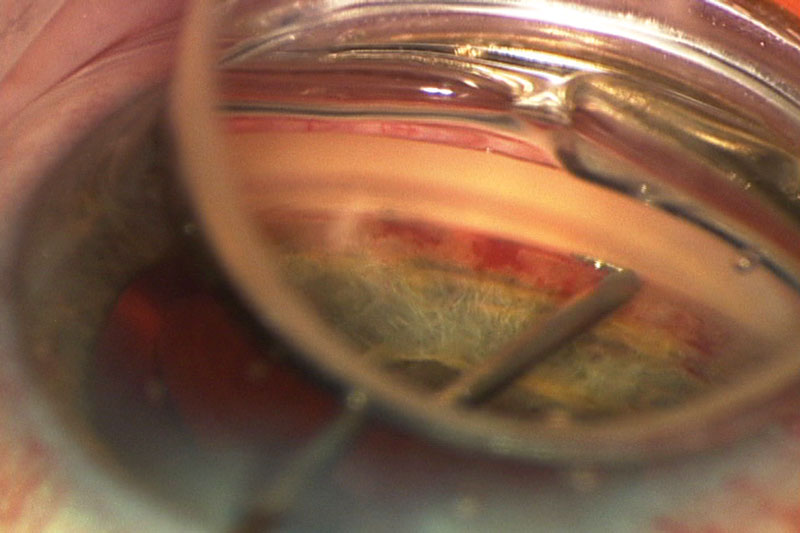 |
| Fig. 2. Under magnification, an iStent is being inserted through the trabecular meshwork during cataract surgery. Photo by Joseph W. Sowka, OD, and Alan G. Kabat, OD |
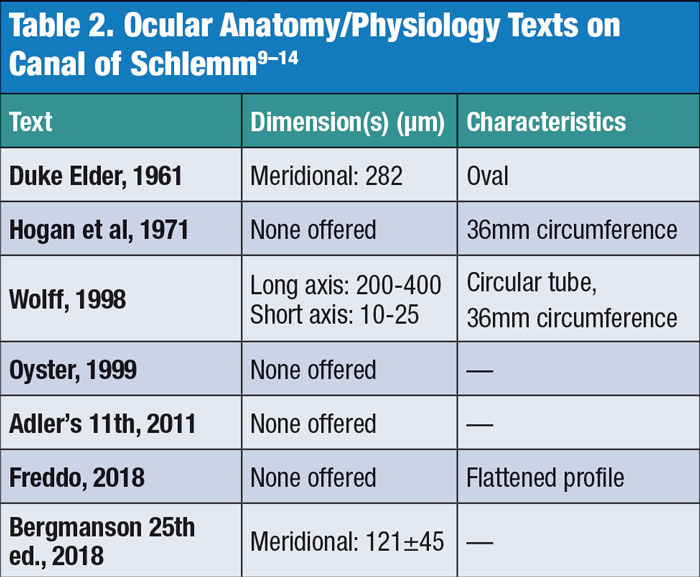
Anatomical effects. Beyond issues with consistency in clinical outcomes, these procedures are performed in the filtration angle, possibly without a clear understanding of the true morphology of the limbal region, the canal of Schlemm and the trabecular meshwork. The exact dimensions and flexibility of Schlemm’s canal remain obscure, with some sources suggesting a range between 141µm and 400µm in width (Table 2).4,14-19 While manufacturers propose that MIGS be placed within the canal of Schlemm, it is possible that this may not consistently occur. (Figures 1 and 2). Though anterior segment OCT scans can show stent penetration into Schlemm’s canal, these are 2D images that make such a determination more difficult.
Adding weight to such concern, stent malpositioning is a known implantation complication for many MIGS procedures, with incidence in literature ranging from 3% to 17.6%.10 In rare cases, clinicians have been unable to locate the stent with gonioscopy after its placement. Though these complications do not lead to severe adverse events, they suggest that positioning may be an unappreciated issue. However, there is a lack of criteria defining malpositioning of the stent, as well as a lack of guidelines for the precise and correct implantation based on anatomical reference points.
Even with correct positioning, successful bypass into the canal of Schlemm is, to our knowledge, not yet confirmed. Gonioscopy in conjunction with biomicroscopy may not produce the magnification and resolution to make a definitive determination of a successful insertion in all cases. The ports of the stent may simply drain into the extracellular tissues external to the canal of Schlemm because of the lack of uniformity in the distance between the internal surface of the trabecular meshes and the internal wall of canal of Schlemm.
Consider the difficulty posed by the triangular shape of the trabecular meshes, where the base is located peripherally adjacent to the scleral spur and the apex is centrally next to the cornea.14 Measurements in our laboratory have indicated that the base of this triangle is approximately 228.26µm and the apex is less than 5µm. The depth to which one needs to insert the catheter to find and penetrate the canal of Schlemm will vary, depending on where in the trabecular mesh the surgeon aims. Many have measured the mesh along its posterior surface facing the anterior chamber at 794.35µm wide; the canal of Schlemm typically only covers half of this width, making successful insertion of a MIGS a surgical challenge.
 |
In addition, a recent pilot study conducted in our laboratory on human cadaver eyes revealed that the canal of Schlemm has an oval shape with its long axis parallel to the ocular surface and its short axis perpendicular to the plane of the cornea (Figure 3). It measured 335.75µm by 33.26µm and appeared to extend across approximately half the width of trabecular meshes. However, in the anterior-posterior axis, our measurement of the lumen opening was 33.26µm, too narrow to be able to physically accept even the smallest stent at 200µm.
A canal of Schlemm filled with aqueous, we predict, may expand to a range of 50µm to 70µm, which will not accommodate a stent either. In such cases, a stent could simply bypass the trabecular meshwork, juxtacanalicular tissue and canal to empty the aqueous into the outer coat external to the canal of Schlemm. Though the trabecular mesh itself has a degree of flexibility, its outer coat anterior to the canal is relatively inflexible by comparison. These uncertainties argue for additional research targeted at mechanism-of-action in vivo.
Possible Corneal Effects
MIGS procedures have a beneficial effect on IOP, though an understanding of their mechanism remains incomplete. Given our current research, some authors suspect these devices deliver the aqueous to the outer coat external to the canal of Schlemm, which could elevate the potential for impact on the cornea. In this location, no membrane or barrier exists to impede the continuous aqueous outflow that pressure differences dictate may allow the rich scleral and conjunctival vasculature to absorb aqueous. This pathway may function similarly to the uveoscleral aqueous outflow, where aqueous does not follow a particular vascular route but simply percolates from the anterior chamber filtration angle to the external eye.
 |
Fig. 3. Aqueous outflow facility in a 70-year-old patient. The triangle indicates canal of Schlemm, the asterisk denotes trabecular meshes and the arrow points at the angle recess. Note the triangular shape of the trabecular meshwork, which creates a non-uniform distance between the canal of Schlemm and the anterior chamber. The central (anterior) edge of the trabecular triangle is not present in this view. |
MIGS may also affect the health of the corneal endothelium, given that the trabecular meshes are a peripheral projection of it. Research shows that the endothelium is compromised in patients with glaucoma and that significant endothelial damage can occur with surgical glaucoma shunts.20 However, little is known about how MIGS affects the corneal and trabecular endothelium.
The only currently published study on this particular topic found no significant difference in the change in endothelial anatomy when comparing three groups (cataract surgery alone, cataract surgery alone in a patient with glaucoma and cataract surgery and Hydrus placement in a patient with glaucoma).21 However, the limitation of this study was the short follow-up period of six months. It is possible that more permanent damage could occur in the already fragile endothelium of the glaucoma patient.21
Lending credence to this theory is the recent removal of the CyPass device from the market (Figure 4). Alcon voluntarily withdrew the implant due to the COMPASS–XT study results showing a statistically significant difference in endothelial cell loss five years post-implantation in patients who had the CyPass implant during cataract surgery compared with those who only had cataract surgery.22 The company has released a statement requesting ophthalmologists discontinue use and return unused devices.22 The company plans to communicate directly with surgeons for the continued management of patients who had previous implantation of the CyPass.22
Given that a suprachoroidal stent may be more invasive than a trabecular stent, this new discovery is a clinical indication of plausible future complications that may be revealed by continued long-term evidence-based research on the effects of MIGS on the corneal endothelium and limbal anatomy. One study noted that when two or more retention rings on the CyPass stent could be observed in the anterior chamber, endothelial cell loss was more significant, though this information may be anecdotal, as there is no citation.23 If this is true, it may suggest that a more standardized method of insertion and implantation could decrease possible endothelial risks.
Additionally, more research regarding the differential segmental outflow of aqueous in the trabecular meshwork, inner wall endothelium of canal of Schlemm, and the episcleral veins is needed to better understand MIGS implantation into the limbal angle.5 One study indicates that more outflow is observed in the inferior and nasal quadrants of the trabecular meshwork because of a greater number of collector channels and an expanded trabecular meshwork in this area.5
 |
Fig. 4. The CyPass stent was recently removed from the market due to endothelial cell loss provoked by the procedure. In this view, a cyclodialysis cleft can be observed around the edges of the stent. Photo by Justin Schweitzer, OD |
While outflow was once thought to be uniformly distributed, it appears now that preferential outflow may exist. One study of bovine eyes associated higher IOP levels with a more contained pathway of resistance to outflow at the areas closest to the collector channel ostia.24 Though this research is interesting, the bovine anatomy of aqueous outflow is different than the human anatomy, as there is no Schlemm’s canal but instead the aqueous plexus, a suggested equivalent counterpart.25 Indeed, one study performed a complete trabeculotomy in enucleated human eyes and found a majority, but not a total reduction in resistance: 50% at a “normal” IOP of 7mm Hg and 75% at a higher IOP of 23mm Hg.
These results indicate that other areas must also contribute significant outflow resistance within the aqueous outflow pathway, though the trabecular meshwork remains a key player in resistance, especially in high IOP situations.24 A better understanding of the aqueous outflow process could help surgeons place these devices more precisely to circumvent the areas of greatest resistance to aqueous outflow. Modifying and standardizing placement could create the potential for greater and more consistent efficacy in the achieved hypotensive effect.
Although MIGS treatments have gained popularity among providers, continued long-term studies of stand-alone MIGS procedures and continued research into the relevant limbal anatomy could help practitioners develop a clearer understanding of the mechanisms. We hope that research will provide an accurate anatomical basis for the intended placement and desired caliber of implants in order to help further the potential success of these MIGS procedures.
Dr. Riccobono is a Cornea and Contact Lens Fellow at the University of Houston College of Optometry, her almer mater.
Dr. Bergmanson is a professor of optometry at the University of Houston College of Optometry, where he is the director of the Texas Eye Research and Technology Center. He is a Foundation Fellow of the College of Optometry in the United Kingdom and a diplomate in the Cornea and Contact Lens section of the American Academy of Optometry.
Mr. Anderson is currently a third year student at the University of Houston College of Optometry.
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthamol. 2006;90(3):262-7. 2. Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-3. 3. Bartlett JD, Fiscella RG, Jaanus SD, Barnebey H. Ocular hypotensive drugs. In: Bartlett, Jaanus eds. Clinical Ocular Pharmacology. Butterworth Heinemann Elsevier; 2008:139-174 4. Dawson DG, Ubels JL, Edelhauser HF. Cornea and sclera. In: Levin LA, Nilsson SFV, ver Hoeve J, et al. Adler’s Physiology of the Eye. 11th ed. Edinburgh: Saunders/Elsevier; 2011. 5. Cha EDK, Xu J, Gong L, Gong H. Variation in active outflow along the trabecular outflow pathway. Exp Eye Res. 2016;146:354-60. 6. Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of schlemm—an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmologica.1972;50(3):295-320. 7. Chen DZ, Sng CCA. Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017;2017:3182935. 8. iStent inject Trabecular Micro-Bypass System (Model G2-M-IS) – P170043. United States Food and Drug Administration. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm612792.htm. Updated July 13, 2018. Accessed October 1, 2018. 9. Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: a meta-analysis. PLoS ONE. 2015;10(7):e0131770. 10. Resende AF, Patel NS, Waisbourd M, Katz LJ. iStent trabecular microbypass stent: an update. J Ophthalmol. 2016;2016:2731856. 11. Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni R. Evaluation of a trabecular meshwork micro-bypass stent in pseudophakic patients with open angle glaucoma. J Glaucoma. 2016 Nov;25(11):896-900. 12. Katz LJ, et al. Long-term titrated IOP control with one, two or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262 13. Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON Study. Ophthalmology. June 23, 2018. [Epub ahead of print]. 14. Bergmanson JPG. Limbus and filtration angle. In: Bergmanson JPG, ed. Clinical Ocular Anatomy and Physiology. 25th ed. Houston: Texas Eye Research and Technology Center, Houston Texas;2018. 15. Duke-Elder S, Wybar KC. The cornea. In: Duke-Elder S, Wybar KC, eds. System of Ophthalmology. Vol 2: The Anatomy of the Visual System. St Louis: Mosby;1961. 16. Hogan MJ, Alvarado JA, Weddell JE. The cornea. In: Hogan MJ, Alvorado JA, Weddell JE, eds. Histology of the Human Eye. Philadelphia: WB Saunders;1971. 17. Bron AJ, Tripathi RC, Tripathi BJ. The cornea and sclera. In: Bron AJ, Tripathi RC, Tripathi BJ, eds. Wolff’s Anatomy of the Eye and Orbit. 8th ed. London: Chapman & Hall Medical;1977. 18. Oyster CW. The cornea and the sclera. In: Oyster CW, ed. The Human Eye: Structure and Function. Sunderland: Sinauer Associates;1999. 19. Freddo TF, Chaum E. The anatomy of the aqueous outflow pathways. In: Freddo TF, Chaum E , eds. Anatomy of the eye and orbit: the clinical essentials. 1st ed. Philadelphia: Wolters Kluwer; 2018. 20. Janson BJ, Alward WL, Kwon YH, et al. Glaucoma-associated corneal endothelial damage: A Review. Surv of Ophthalmol. 2018;63(4):500-6. 21. Fea AM, Consolandi G, Pignata G, et al. A comparison of endothelial cell loss in combined cataract and MIGS (Hydrus) procedure to phacoemulsification alone: 6-month results. J Ophthalmol. 2015;2015:769289. 22. Alcon Announces Voluntary Global Market Withdrawal of CyPass Micro-Stent for Surgical Glaucoma. United States Food and Drug Administration. www.fda.gov/Safety/Recalls/ucm619109.htm. Updated August 29, 2018. Accessed October 1, 2018. 23. Sng CCA, Barton K. Minimally invasive glaucoma surgery – coming of age. Br J Ophthalmol 2018;102:1315-6. 24. Battista SA, et al. Reduction of the available area of aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes, Invest Ophthalmol Vis Sci.2008;49(12): 5346–52. 25 Overby D, Gong H, Qiu G, Freddo T, Johnson M. The Mechanism of Increasing Outflow Facility during Washout in the Bovine Eye. Invest Ophthalmol Vis Sci.2002;43:3455-64. |

