 Occasionally, we encounter a drug that is prized just as muchor morefor its side effects as it is for its original indication. For example, Benadryl (diphenhydramine, Johnson & Johnson) is a powerful antihistamine indicated for the relief of sneezing, watery eyes and itchy throat due to allergies. But, this drug is also well known for its ability to cause drowsiness. So, we also have products today like Nytol (diphenhydramine, GlaxoSmithKline) and Tylenol PM (acetamino- phen/diphenhydramine, McNeil PPC) that incorporate diphenhydramine primarily as a sleep aid rather than an antihistamine.
Occasionally, we encounter a drug that is prized just as muchor morefor its side effects as it is for its original indication. For example, Benadryl (diphenhydramine, Johnson & Johnson) is a powerful antihistamine indicated for the relief of sneezing, watery eyes and itchy throat due to allergies. But, this drug is also well known for its ability to cause drowsiness. So, we also have products today like Nytol (diphenhydramine, GlaxoSmithKline) and Tylenol PM (acetamino- phen/diphenhydramine, McNeil PPC) that incorporate diphenhydramine primarily as a sleep aid rather than an antihistamine.
Another example: the popular drug Viagra (sildenafil citrate, Pfizer). You probably recognize this medication as a treatment for erectile dysfunction, but you may not recall that sildenafil was initially developed and formulated as an anti-hypertensive medication.1
It is indeed intriguing that these two products, originally designed to overcome illness, have become more widely utilized as a means of improving quality of life. Until very recently, optometrists did not have much cause to worry about medication abuse or gross off-label usage. Of course, there have always been stories about those troublesome patients who purloin a bottle of proparacaine to self-treat their corneal abrasion or continue to use their TobraDex ointment (tobramycin/dexamethasone, Alcon) to help alleviate puffy eyelids. But for the most part, ophthalmic medications do not have a great tendency for misuse.
Recently, however, one off-label application of a popular glaucoma drug has generated a great deal of interest among researchers, clinicians and entrepreneurs.
Medical Mascara?
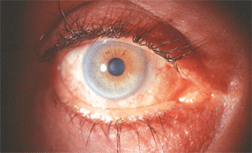
Eyelash hypertrichosis in a patient using topical bimatoprost for glaucoma. Some health-care professionals are prescribing the drug off-label for this purpose.
Image courtesy: Barry J. Frauens, O.D., Nova Southeastern University
Today, prostaglandin analogs are probably the most commonly prescribed glaucoma agents in the
The more common ocular complications, however, are largely cosmetic in naturenamely, conjunctival hyperemia, increased iris pigmentation, periocular skin darkening and hypertrichosisan increased thickening, lengthening and darkening of the eyelashes.6-10 It is this last side effect of topical prostaglandins that has gained national attention in recent months.
In November 2007, an article in The Wall Street Journal reported that thousands of tubes of Age Intervention Eyelash (Jan Marini Skin Research, Inc.), valued at more than $2 million, had been seized by federal agents acting on orders of the U.S. Food and Drug Administration (FDA). Reportedly, the product contained bimatoprost, the active ingredient in Lumigan (Allergan). In a statement, the FDA chastised Jan Marini Skin Research, referring to their cosmetic eyelash treatment as an unapproved and misbranded drug.
Lumigan received FDA approval in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.11 The FDA suggested that patients who use bimatoprost cosmetically in conjunction with topical bimatoprost may be at risk for increased optic nerve damage because the extra dose would, in fact, decrease the drugs effectiveness. Additionally, the FDA cited the potential for other adverse effects in certain people using the product, including macular edema and uveitis.
Allergan has since filed a patent infringement lawsuit against Jan Marini Skin Research and six other companies that are allegedly selling eyelash-growth products that contain bimatoprost. To date, Allergan has not commented publicly regarding this matter. However, Heather Katt, senior manager of corporate communications for Allergan, told us, Allergan is very aggressive in protecting its intellectual property against those who would attempt to trade on its hard work and intellectual property portfolio Anyone who takes an active drug ingredient and then concocts a product to sell for eyelash growth is violating FDA law by selling an unregulated and unapproved drug that has not been subjected to FDA review or the rigorous safety and quality assurance requirements associated with pharmaceutical manufacturing.
The question remains: Does the use of topical prostaglandins benefit those individuals seeking longer, thicker and more attractive eyelashes? A study is currently underway at the
Some physicians are convinced that further studies are unnecessary and have been advocating the use of drugs like Lumigan in this capacity to their patients. One such physician is Lorrie Klein, M.D., a dermatologist in
From an optometric point of view, this is very thought provoking. If patients are using ophthalmic products off-label as a cosmetic, who is better qualified to prescribe for and monitor these individuals? A dermatologist, who is less familiar with possible ocular complications, or an optometrist? Who has the ability to provide a comprehensive ocular examination prior to initiating treatment to ensure that there are no specific contraindications?
This is not to suggest that optometry suddenly embark on a collective exploitation of products and services for patients cosmetic benefit. But, such events should prompt us to realize that other health-care professionals routinely go beyond the specific indications outlined in a given products package insert.
In addition, optometrists need to be clear on what constitutes off-label use, and the FDAs position on such action. Many practitioners are surprised to realize that off-label prescribing does not specifically violate FDA law. According to information sheets provided by the FDA, a physician may use a product for an indication not in the approved labeling when the intent is the practice of medicine, and is based on firm scientific rationale and sound medical evidence.14
The decision to prescribe topical prostaglandins for cosmetic purposes is one that must be made based upon the individual needs of the patient and the associated risks involved. Optometrists have the responsibility to provide appropriate and comprehensive care; in some cases, this may include off-label use of ophthalmic products, but only if it is done with the most altruistic of intentions.
1. Kling J. From hypertension to angina to Viagra. Mod Drug Discov 1998;1(2):31,33-34,36,38.
2. Inan UU, Ermis SS, Orman A, et al. The comparative cardiovascular, pulmonary, ocular blood flow, and ocular hypotensive effects of topical travoprost, bimatoprost, brimonidine, and betaxolol. J Ocul Pharmacol Ther 2004 Aug;20(4):293-310.
3. Hedner J, Everts B, Mller CS. Latanoprost and respiratory function in asthmatic patients: randomized, double-masked, placebo-controlled crossover evaluation. Arch Ophthalmol 1999 Oct;117(10):1305-9.
4. Suominen S, Vlimki J. Bilateral anterior uveitis associated with travoprost. Acta Ophthalmol Scand 2006 Apr;84(2):275-6.
5. Wand M, Shields BM. Cystoid macular edema in the era of ocular hypotensive lipids. Am J Ophthalmol 2002 Mar;133(3):393-7.
6. Feldman RM. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J Ocul Pharmacol Ther 2003 Feb;19(1):23-35.
7. Stjernschantz JW, Albert DM, Hu DN, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol 2002 Aug;47 Suppl 1:S162-75.
8. Herndon LW, Robert D Williams, Wand M, Asrani S. Increased periocular pigmentation with ocular hypotensive lipid use in African Americans. Am J Ophthalmol 2003 May;135(5):713-5.
9. Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol 1997 Oct;124(4):544-7.
10. Parrish RK, Palmberg P, Sheu WP; XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003 May;135(5):688-703.
11. Allergan, Inc. Lumigan package insert, 2001.
12. Efficacy study of latanoprost and bimatoprost solutions in promoting eyelash growth in patients with alopecia areata. J Drugs Dermatol 2006 Jan;5(1):95.
13. Klein L. Lumigan Eyelash Extension. 2007. Available at: www.skin1.com/lumigan.html (Accessed February 2008).
14. IRB Information Sheets. Off-Label and Investigational Use of Marketed Drugs, Biologics, and Medical Devices. 2001. Available at: www.fda.gov/oc/ohrt/irbs/offlabel.html (Accessed February 2008).

