Low vision care is a crucial part of our profession. It’s endorsed by many key organizations, including the American Academy of Optometry, the American Optometric Association, the American Academy of Ophthalmology, the National Eye Institute and the World Health Organization. The American Academy of Ophthalmology’s CEO, David Parke, MD, even claimed in a recent video that “vision rehabilitation is now the standard of care for patients who are losing their vision.”1 This is a strong message: if you’re not referring for low vision care, you’re not practicing at an acceptable standard.
Most optometrists eventually refer patients in need of low vision services, but the process for determining when to refer is often up for debate. The most common approach is to set a visual acuity threshold; many refer for low vision care when best-corrected visual acuity in the better-seeing eye reaches 20/40, 20/60 or 20/70. Some educational and vocational programs even set a visual acuity threshold for admission.
However, basing your low vision referral on visual acuity alone is insufficient and ignores many other visual complications that decrease patients’ visual functioning and quality of life. For example, visual acuity may be minimally affected, but a deficiency in contrast sensitivity, visual field, ability to handle glare or see at night, color vision, stereopsis or binocularity can affect a patient’s functioning equal to that of visual acuity loss.
So, if you can’t depend on visual acuity to guide your referral decision, what can you base it on? My preferred definition of low vision comes from the National Eye Institute: “Low vision is a vision problem that makes it hard to do everyday activities. It can’t be fixed with glasses, contact lenses or other standard treatments like medicine or surgery.”2 So, when a patient’s life is impacted by vision, it’s time to refer, regardless of visual acuity.
This article provides a more comprehensive approach to low vision referrals with the help of several case examples. The cases, although incomplete, provide vignettes of relevant information to highlight the many different patient populations that could benefit from low vision services—and it’s more than you might have realized.
 |
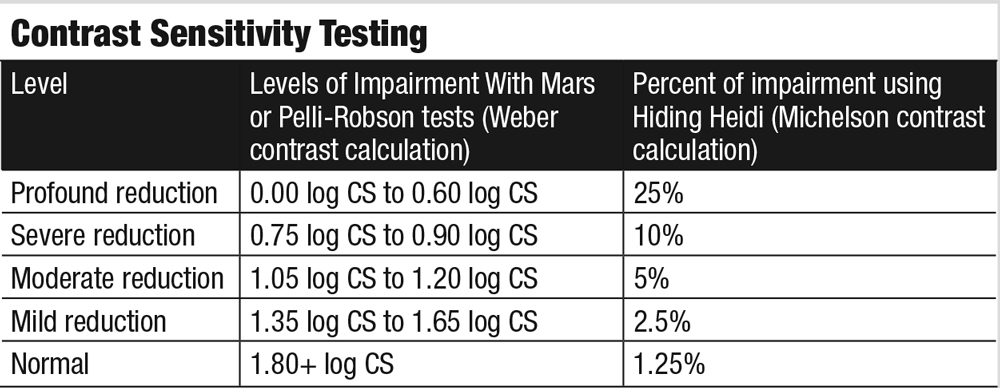
| Fig. 1. This microperimetry readout shows a case of a spared channel of central vision in a patient with macular telangiectasia. Click image to enlarge. |
Foveal Sparing
Some patients with age-related macular degeneration (AMD), especially those with central geographic atrophy from non-exudative AMD, develop a large central scotoma with a tiny island of vision at the center. These types of patients may have 20/30 vision when reading letter-by-letter on a visual acuity chart, but that does not reflect their visual function. When reading in a real-world environment, they may function more like a patient with visual acuity ten times worse.
Testing near vision with a well-designed continuous text chart, such as the MNRead or the SK Read is often quite revealing, as these tests can expose a much deeper level of visual impairment than a visual acuity chart. Inability to fluently read a 1.0M sentence with ordinary glasses is an indication that referral might be beneficial.
Another important sign of foveal sparing is the paradoxical situation of greater ease with reading smaller rather than larger print, because the small island of central vision is incapable of taking in more than a letter or two at a time when the text is large.
Bright illumination can be an effective tool in managing patients with foveal sparing, as the portions of relative scotoma will begin to contribute to the visual process if lighting is sufficient, effectively limiting the size and scope of the ring scotoma.
A sure sign of a patient with foveal sparing is the patient who describes walking outside into bright sunlight to read print or keeping a portable flashlight with them everywhere they go.
Microperimetry can be another useful tool for investigating foveal sparing. Figure 1 shows a patient with macular telangiectasia and reveals a narrow corridor of dim vision just a few degrees wide, surrounded by a large area of blindness nearly 20 degrees in diameter. She was able to register 20/50 on an ETDRS visual acuity chart, although this, in no way, represents how she functions in the real world. She benefitted from bright illumination for some tasks, electronic magnification for reading and occupational therapy for assistance with many activities of daily living.
Contrast SensitivityFor adults, the Pelli-Robson or Mars tests are quick and convenient ways to evaluate contrast sensitivity. Although only one spatial frequency is evaluated, they provide useful data and a great opportunity to educate patients. The Hiding Heidi test is remarkable in assessing pediatric patients as young as two years. Functional problems become evident when contrast sensitivity reaches the moderate or severe level of reduction from normal.
|
Contrast Sensitivity Loss
Contrast sensitivity is extremely important in determining how a person functions. It’s critical in distinguishing the flesh tones we use to identify a face and in determining where a sidewalk meets a curb or a step when walking. For a person with very poor contrast sensitivity, seeing rice on a white plate or coffee in a black cup becomes nearly impossible.
The level of contrast sensitivity loss is not predicable based on visual acuity. Consider these real-life examples, in which the patient’s contrast sensitivity loss is not at all proportionate to their visual acuity deficit:
A 90-year-old female with AMD had visual acuity of 20/80 but log contrast sensitivity of 0.45, which is considered profound loss
An 84-year-old male with AMD had visual acuity of 20/400 and log contrast sensitivity of 1.50, which is only mild loss
An 83-year-old female with AMD had visual acuity of 20/40 and log contrast sensitivity of 0.75, which is severe loss
As these examples illustrate, contrast sensitivity testing can reveal a level of vision loss that would not be predicted by visual acuity alone.
The 90-year-old female described above was an avid mahjong player but had to stop playing due to her vision loss. She was able to resume playing after I fit her with a 3x Ocutech Mini variable focus telescope, mounted to use in slight downgaze and in good illumination (Figure 2).
Visual Field Loss
A patient can have 20/20 visual acuity alongside many conditions that cause visual field loss such as: homonymous hemianopia, bitemporal hemianopia, concentric loss, paracentral scotoma, ceco-central scotoma and altitudinal loss.
Due to this variety of visual field loss, it is impossible to provide a specific criterion on which to base a low vision referral. Rather, if your patient has visual field loss, refer for low vision care if the loss interferes with their daily activities. Scanning training, reverse telescopes, Peli prisms and improved illumination can assist patients with problems related to visual field loss. Depending on their vision and their personal needs, we may refer the patient for orientation and mobility training and, where appropriate, behind-the-wheel driving rehabilitation services.
 |
| Fig. 2. We used a 3x Ocutech mini variable focus telescope to help this patient see when playing mahjong. Click image to enlarge. |
Diplopia
Many conditions can cause double vision; for example, it is a sequela of glaucoma drainage device implantation in about one in four cases.3,4 Diplopia and the resulting binocular confusion can be difficult problems for both the patient and the clinician. One reason is that suppression rarely provides relief for adult patients, given their relatively lower level of neuroplasticity and ability to suppress compared with children. Prism glasses, referral for strabismus surgery and a wide spectrum of options for occlusion exist to assist patients with diplopia. Consider these two patient examples:
An 80-year-old female had experienced extremely frustrating constant diplopia for more than two years secondary to thyroid eye disease with orbital decompression surgery. Her visual acuity was corrected to 20/32 in the right eye and 20/40 in the left. She had a 14Δ left hypertropia that increased significantly in left gaze. She was fit with progressive addition lenses with +3.00D add and prism of 6Δ base-up in the right eye and 6Δ base-down in the left. She was thrilled to have her diplopia controlled with the glasses in all but far left gaze.
Sometimes, one pair of glasses can’t solve all of a patient’s problems. A 38-year-old female presented with Kearns-Sayre syndrome, a mitochondrial condition that can cause symptoms similar to retinitis pigmentosa, such as nyctalopia and mid-peripheral visual field loss. Another common sign of this syndrome, experienced by this patient, was external ophthalmoplegia. She was orthophoric at distance and had the accommodation expected of someone her age but had no ability to converge. Single vision computer glasses were provided with +0.50D add and a total of 7Δ base-in. For reading, she received a +1.25D add with a total of 13Δ base-in, also in single vision lenses.
20/UnhappyIn my experience, referral sources sometimes send patients who are not technically low vision, yet they are unhappy with their vision. By far the most common type of referral in this category is from cataract surgeons, who have completed a cataract extraction with intraocular lens (IOL) implantation, and the patient is unhappy with their vision despite registering normal visual acuity of 20/20 or 20/25 on standard visual acuity tests. This is more common with, but not limited to, the use of multifocal IOLs. A significant number of multifocal IOL patients have more higher-order aberrations and slightly reduced contrast sensitivity compared with patients with monofocal IOLs.1,2 Dissatisfaction with multifocal IOLs is somewhat dependent on personality type.3 Here is a recent example: A 71-year-old retired accountant was referred by her cataract surgeon. She had received Tecnis Symfony (Johnson & Johnson Vision) multifocal IOLs with her cataract surgery six months previously. She was dissatisfied with her vision for reading and night driving. I was given a tall order from the cataract surgeon: come up with a plan to improve her vision without prescribing glasses because she didn’t want them (hence the multifocal IOLs). Her left eye was strongly dominant. She refracted to 20/15 in each eye with plano -0.50 x 040 in the right eye and +0.25D sphere in the left. My recommendation, which the cataract surgeon carried out, was to perform PRK on the right eye to leave it at -0.75D sphere and use brimonidine drops OU when driving at night to slightly reduce pupil size. These changes were successful, but she did finally consent to wearing glasses with -0.75D sphere for the right eye when driving at night.
|
Acute Monocular Vision Loss
An estimated two to four in 100,000 people per year will experience enucleation or evisceration of one eye, and one study found 2% of US veterans in a polytrauma center were monocular.5-8
Adjusting to acute vision loss in one eye can be a monumental task, and it is especially helpful to refer when the loss is recent and occurred suddenly, as in a case of ocular injury or enucleation from ocular malignant neoplasm.9,10 Any type of vision rehabilitation program, including those in private practices, academic institutions, non-profit clinics and the Veterans Administration, can help patients adapt to acute monocular vision loss. The following cases illustrate the benefits these programs provide:
A 46-year-old marketing specialist had her left eye enucleated due to malignant melanoma. The right eye had normal visual acuity, visual field, contrast sensitivity and color vision. She was quite symptomatic from the loss of the temporal crescent of visual field in the left eye and reported mobility difficulties.
The level of difficulty from the loss of visual field is often disproportionate to the amount of actual visual field loss from monocularity in many patients. Other symptoms also difficult to explain include the need for more light, photophobia and fatigue with visually intense tasks. As expected, this patient also suffered a complete loss of stereopsis and had difficulty with hand-eye tasks such as activities involving reach-and-grasp ability and pouring liquids from a pitcher.
Following her low vision evaluation, we referred her to our occupational therapist, who helped her adapt with new strategies for daily tasks, such as hand-eye activities and training to develop a method for scanning to her left when walking.
Partial monocular vision loss also causes troublesome rivalry symptoms for some patients. A 78-year-old retired physician and professor had significant visual field loss and reduced contrast sensitivity in the left (formerly dominant) eye due to glaucoma. His symptoms were so severe he told me he felt his vision would be better if he lost the left eye entirely, and he often closed the left eye when performing visually intense tasks. His visual acuity was correctable to 20/32 in the right eye and 20/50 in the left.
After several visits, we determined different levels of occlusion would help for different tasks. For some activities he required total occlusion of the left eye, but for others he preferred partial occlusion, which we provided with Bangerter foil. We prescribed single vision lenses for mobility with 0.2 Bangerter foil over the entire left lens. For reading, we placed 0.2 Bangerter foil over the bifocal portion only of his left lens and included a +3.00D bifocal add in the right lens. For computer use, he required total occlusion, achieved with a left lens painted black and a single vision lens for the right eye with a +1.50D add.
Low Vision MetricsIf you’re determined to attach numbers to the decision-making process, I suggest following the 40-20-1 rule from Roy Cole, OD, now retired and one of the most respected and beloved people in low vision. Dr. Cole likes to say a person should be referred for low vision care if:
Dr. Cole also developed an eight-question screening protocol to help identify patients in need of low vision services. I have added an item to Dr. Cole’s list (#7 below) that is far more relevant today. Do you have trouble doing what you want to do because of your vision? For example, do you have difficulty:
During the past month, have you, due to your loss of vision: 8. Been feeling down, depressed or hopeless? 9. Had little interest or pleasure in doing things? If the patient answers yes to any of these questions and refractive, medical or surgical strategies cannot help, it’s time to refer for low vision.1
|
Night Blindness
Nyctalopia occurs most famously in retinitis pigmentosa, which affects nearly 100,000 people in the United States, but it can occur with other conditions as well.11 Again, visual acuity may not be bad enough to trigger a referral, but a person can be significantly impaired from night blindness and could benefit from low vision care.
For many of our retinitis pigmentosa patients, we recommend a high-end flashlight, such as the MT 14 by LED Lenser. At 1,000 lumens, it is extremely bright, has a uniform, adjustable wide field of illumination, is rechargeable and maintains full brightness as the battery discharges. It might seem excessive to spend more than $100 for a flashlight, but it’s significantly better than what is often purchased in a hardware or discount store. Many of our patients report that they frequently use the flashlight on their smart phone, but this provides 100 lumens or less, and the light is diffuse rather than focused. The difference between the phone flashlight and the MT 14 is easy to appreciate for almost all of our patients with nyctalopia.
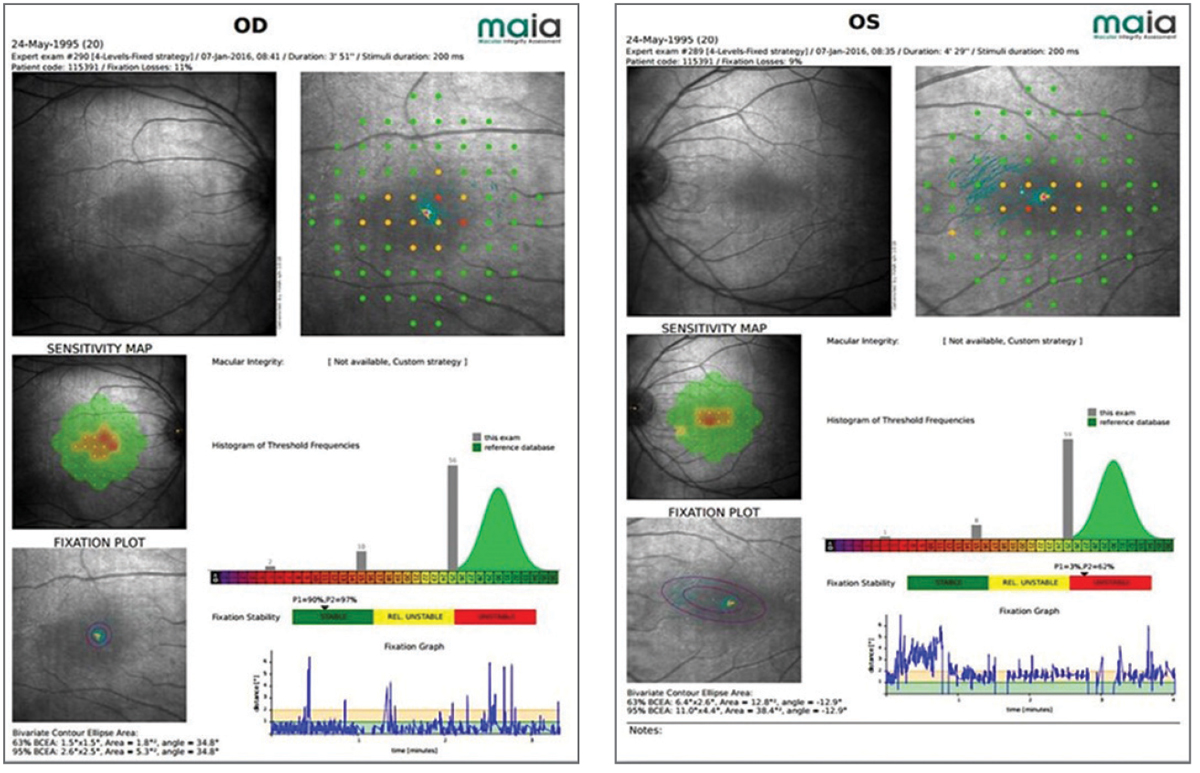 |
| Fig. 3. Microperimetry of a 20-year-old patient with partial achromatopsia reveals small central scotomas that affect his vision. Click image to enlarge. |
Photophobia
Cone dystrophies, achromatopsia, aniridia, oculocutaneous and ocular albinism are just some of the conditions that can result in extreme photophobia. In many cases, these patients would qualify for a low vision referral based on visual acuity, but addressing the photophobia by selectively attenuating light with filters can greatly assist these patients. For reasons we only partially understand, patients with cone dystrophies and achromatopsia frequently report far less photophobia and greater visual comfort and ability to see detail when using filters in the red portion of the spectrum.12 Red-tinted contact lenses are a viable and more discreet option for these patients.13-15 This patient’s experience illustrates the benefits of many different low vision strategies, including management of photophobia:
A 20-year-old computer science student presented with incomplete achromatopsia, left esotropia and amblyopia. He was referred for a host of vision related challenges, including extreme photophobia. His best-corrected visual acuity was 20/60 in the right eye and 20/80 in the left. He showed moderate contrast sensitivity loss and suppression of the left eye on Worth four-dot test. Microperimetry indicated small central scotomas (Figure 3). He was provided with a pocket magnifier and single vision glasses with a 35% transmission red tint. Happy with the results, he later returned for a 2.2x bioptic telescope with the same red tint in both the carrier lens and the telescope.
The breadth of cases we work with in low vision rehabilitation is quite remarkable. Rather than depending on a specific level of visual acuity loss to guide your decision about referral, simply talk to your patients and find out if they’re experiencing functional problems due to their vision. If so, referral to a low vision rehabilitation specialist might be helpful. Your patients will thank you when they return for ongoing care.
Dr. Lewerenz is an assistant professor at the University of Colorado School of Medicine, Department of Ophthalmology. He provides low vision services at the UCHealth Sue Anschutz-Rodgers Eye Center. He is a clinical diplomate in Low Vision in the American Academy of Optometry. He has no conflicts of interest that relate to this article.
| 1. American Academy of Ophthalmology. The Academy’s Initiative in Vision Rehabilitation. www.aao.org/low-vision-and-vision-rehab. Accessed November 18, 2019. 2. National Eye Institute. Low Vision. www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/low-vision. Accessed November 18, 2019. 3. Sun PY, Leske DA, Holmes JM, Khanna CL. Diplopia in medically and surgically treated patients with glaucoma. Ophthalmology. 2017;124(2):257-62. 4. Islamaj E, Jordaan-Kuip CP, Vermeer KA, et al. Motility changes and diplopia after Baerveldt glaucoma drainage device implantation or after trabeculectomy. Trans Vis Sci Tech. 2018;7(5):7. 5. Trawnik WR, Fitzsimmons TD. Eye loss in the 1990s: A comparative study. J Ophthal Pros. 1996;2:1-14. 6. Erie JC, Nevitt MP, Hodge D, Ballard DJ. Incidence of enucleation in a defined population. Am J Ophthalmol. 1992;113:138-44. 7. Sigurdsson H, Thórisdóttir S, Björnsson JK. Enucleation and evisceration in Iceland 1964-1992. Study in a defined population. Acta Ophthalmol Scand. 1998;76(1):103-7. 8. Cockerham GC, Goodrich GL, Weichel ED, et al. Eye and visual function in traumatic brain injury. J Rehabil Res Dev. 2009;46(6):811-8. 9. Ihrig C. Vision rehabilitation team management of acquired monocular vision. Optom Vis Sci. 2013;90(3):e89-94. 10. Ihrig C, Schaefer DP. Acquired monocular vision rehabilitation program. J Rehabil Res Dev. 2007;44(4):593-7. 11. National Institutes of Health, US National Library of Medicine. Genetics Home Reference. ghr.nlm.nih.gov/condition/retinitis-pigmentosa#statistics. Accessed November 29, 2019. 12. Haegerstrom-Portnoy G, Schneck ME, Verdon WA, Hewlett SE. Clinical vision characteristics of the congenital achromatopsias. II. Color vision. Optom Vis Sci. 1996;73(7):457-65. 13. Park WL, Sunnes JS. Red contact lenses for alleviation of photophobia in patients with cone disorders. Am J Ophthalmol. 2004;137(4):774-5. 14. Schornack MM, Brown WL, Siemsen DW. The use of tinted contact lenses in the management of achromatopsia. Optometry. 2007;78(1):14-22. 15. Rajak SN, Currie AD, Dubois VJ, et al. Tinted contact lenses as an alternative management for photophobia in stationary cone dystrophies in children. J AAPOS. 2006;10(4):336-39. |