In 2015 in the United States, 3.6 million cataract surgeries were performed, and more than 20 million are performed yearly worldwide.1 Today’s cataract surgery patients have access to new technologies that provide better preoperative and intraoperative surgical precision, which allow for unprecedented refractive outcomes. The rapid evolution in cataract surgery is nothing short of spectacular, and yet, a major drawback remains for our patients: the burden associated with instilling numerous postoperative eye drops.
While cataract surgery is safe, achieving and maintaining such high outcomes requires patients correctly instill eye drops after the procedure—for which no standard currently exists. The potential lack of adherence to the postoperative drop regimen can lead to complications, the most devastating being endophthalmitis.2 Though a rare occurrence, it’s dire enough to warrant consideration of every possible protection against it.
Intracameral (‘into a chamber’) or injectable medications after cataract surgery may be able to help patients overcome the hassle of postop drops and avoid complications.3-5 Here, you’ll find essential details regarding patients who opt for dropless or reduced-drop cataract procedures and an overview of the OD’s critical role in care.
Injectables: Our Protocol
In our practice, we use Tri-Moxi (triamcinolone 15mg/mL + 1mg/mL moxifloxacin, Imprimis) injections for the majority of cataract surgery patients and a ‘drop-a-day’ regimen, which includes topical NSAIDs. Our surgeons have used both trans-zonular and intravitreal Tri-Moxi, and now primarily use the intravitreal route. Dex-Moxi (dexamethasone 0.1% + moxifloxacin hydrochloride 0.5%, Ocular Science) is another compounded intravitreal option that tends to be a clearer steroid, so likely creates less vitreous debris/haze; at the same time, dexamethasone has a higher likelihood of steroid response.
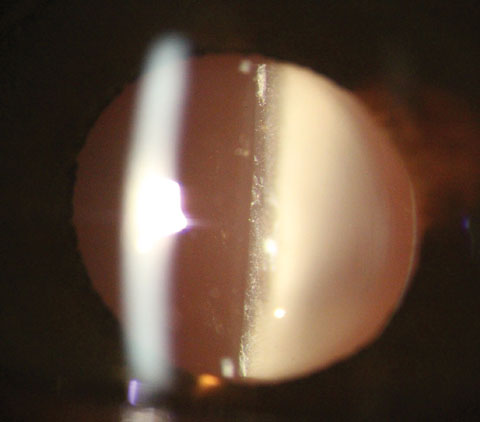 |
| Fig. 1. Presentation one day after dropless cataract surgery using Tri-Moxi. Note the white “snowglobe” appearance in the anterior vitreous common after dropless surgery. Click image to enlarge. |
At the conclusion of cataract surgery, while the patient is still under anesthesia, 0.15cc of Tri-Moxi is injected by intravitreal route. We maintain the importance of the betadine swab prophylactically before surgery. After the procedure, we start patients on a nonsteroidal anti-inflammatory (NSAID) drop once daily for one month. For patients with diabetes, epiretinal membranes with or without macular traction, or other pre-op risk factors for postoperative cystoid macular edema (CME), patients are kept on NSAIDs once daily for three months post procedure.
Fully dropless cataract surgery may work great for some patients, but in our practice we prefer a ‘drop-a-day’ regimen and the anti-inflammatory effects of topical NSAIDs compared with topical steroids. Research directly compares pseudophakic cystoid macular edema (PCME) in post-cataract surgery patients who were randomized to topical NSAIDs or topical steroids to mediate inflammation.6 At one month, 25% of patients in the steroid alone arm had measurable PCME compared with less than 4% in the NSAID alone group.6
Set the Bar
For postoperative intracameral injections, setting expectations is a critical key to success, for both the patient and their clinician.
For the patient, clinicians must tackle the majority of education in the preoperative evaluation. In our practice, we educate patients on two categories of postoperative experiences: mild blurring or haziness of vision for three to four days, and increased floaters and vitreous debris for up to one month. Importantly, we over-emphasize what most patients will encounter. We also educate patients about the increased likelihood of a subconjunctival hemorrhage, especially in patients who take blood thinners, higher doses of aspirin or omega-3 fatty acid supplements.
Clinicians should expect to see a white “snowglobe” appearance in the anterior vitreous hours after the procedure, and into the one-day post-op check (Figure 1). On the one-day postop, clinicians should expect to see visual acuity between 20/20 and 20/100, which should improve similarly to patients prescribed traditional postoperative drops. In our experience, corneal edema, if present, and anterior chamber inflammation both resolve much quicker with intracameral injections compared with a drop regimen. On dilated peripheral exam, the examiner will note a clumping, white material that generally rests in the inferior vitreous.
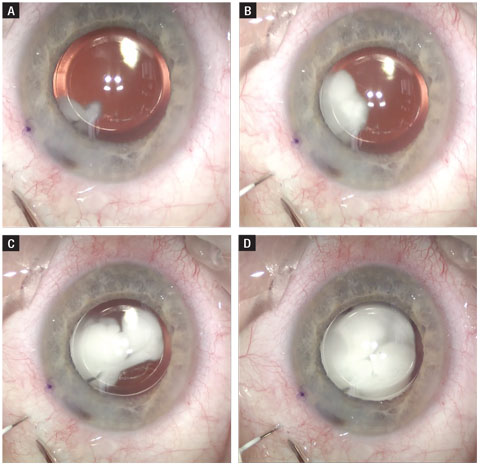 |
| Fig. 2. Here, this time lapse shows an intravitreal injection of triamcinolone and moxifloxacin. The needle is routed posterior to the IOL and has a similar final destination to that of an anti-VEGF injection. Click image to enlarge. |
Three Cs
The advantages of an intracameral injection over traditional drops can be summarized by the three Cs: compliance, cost and complications.
Compliance. Correctly and repeatedly instilling postoperative eye drops is a known difficulty for patients. A recent study shows only two-thirds of patients feel confident in instilling eye drops, and 42% believed they never missed their eye on instillation.7 More concerning, the same study’s objective data shows less than 8% of patients properly instilled the drops per the study protocol, with the most common error being contamination of the bottle.7
Compliance with medications is almost always improved with decreased dosing. Traditional post-op drop regimens often require three bottles and up to 12 drops per day: an antibiotic four times daily for one week, an NSAID once daily for one month, and a steroid four times daily for one week, then tapered to twice daily for one week. Such protocols can be challenging for many patients, especially those with physical limitations such as Parkinson’s disease and rheumatoid arthritis. If the practitioner substitutes this with a single injection of Tri-Moxi plus a once-daily NSAID, the patient’s drop burden decreases by roughly 70 drops per eye.
Cost. The traditional post-op regimen of topical antibiotic, steroid and NSAID can pose a significant cost burden to patients, even those with health insurance. An intracameral injection eliminates the need for the patient to purchase both a topical steroid and antibiotic.
According to one drug price comparison website, the price for one bottle of Vigamox (moxifloxacin HCl ophthalmic solution, Alcon) averages $160, while the same site has a mean price of about $60 for prednisolone acetate 1%.8,9 Tri-Moxi, with an average price of $25 (no cost to patient), potentially saves a patient more than $400 for both eyes. With 1.82 million cataract surgeries performed in the Medicare population alone in 2011, the cost in postoperative antibiotics alone to Medicare would have been $136 million in 2011—and that’s just using the conservative cost estimate of moxifloxacin at $75/bottle.
Currently, no billing code exists for post-surgical injections, so they aren’t reimbursable by insurance. In our practice, we absorb the cost of Tri-Moxi for our patients because we see the immense value to both our patients and practice. Intravitreal/tranzonular medications have significant potential not only to lower the cost of medications for our patients, but also for private insurers and Medicare. We have found the small cost of Tri-Moxi to our organization is offset by less medication call backs from pharmacies, less prior authorizations side effect concerns.
Complications. While patients may benefit from dropless cataract surgery, use of injections after cataract surgery may help surgeons just as much, if not more, by minimizing the risk of postoperative complications, especially endophthalmitis.
Research has found endophthalmitis rates in the range of 0.04% to 0.2% after cataract surgery, and these numbers continue to decrease following a spike in reported cases during the late 1990s and early 2000s.10 In 2006, the European Society of Cataract and Refractive Surgery published a landmark study comparing endophthalmitis rates post-cataract surgery in patients using drops versus intracameral antibiotics.11 This study shows a fivefold decrease in endophthalmitis rates when intracameral cefuroxime is injected at the conclusion of surgery.11
One of the largest comparative studies on topical drops vs. injections, which looked at the result of more than16,000 cataract surgeries, concluded that intracameral cefuroxime with or without topical antibiotics decreased endophthalmitis rates 22-fold.5
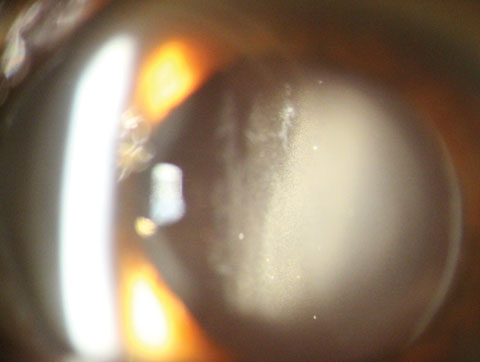 |
| Fig. 3. Evidence of the white debris (medication) in the anterior vitreous one day after cataract surgery. Click image to enlarge. |
The Risks
Although intravitreal and transzonular injections have moved the needle up in our practice, doing what is safest for our patients’ eyes is paramount, and practitioners must be aware of the risks associated with their use.
A recent article reviewed outcomes with transzonular injections vs. topical postop medications and provides important data, though it contained a small number of patients and a larger case series is warranted.12 This contralateral eye study randomized patients to either cataract surgery with drops in one eye or cataract surgery with transzonular Tri-Moxi-Vanc (triamcinolone 15mg + moxifloxacin 1mg + vancomycin 10mg/mL, Imprimis) in the other eye. Here’s what it concluded about the postoperative risks of injectable medications:
Cystoid macular edema. No statistically significant difference in macular thickness at one week and one month post-op existed between the study groups.12
Clinician’s take: We have not observed increased CME rates using intravitreal injections, and we prescribe NSAIDs once daily for one month in routine cases.
IOP spikes. The study did not show statistical significance in IOP spikes or IOP readings between the two groups.12
Clinician’s take: We have studied intravitreal injections vs. drops for IOP spikes in patients who undergo cataract surgery and the insertion of a trabecular microbypass stent (Glaukos). Our data, presented at the American Society of Cataract and Refractive Surgery (ASCRS) meeting in 2016, showed no statistically significant difference in IOP between the two groups.12
Postop pain. The study found a statistically significant difference in pain post-surgery.12 In this cohort, all patients had cell, flare or both in the anterior chamber on day one post-op, but this inflammation resolved over time near equally with patients who used the traditional post-op drop regimen.
Clinician’s take: If corneal swelling is present, it resolves quicker with intravitreal medication, and the absence of anterior chamber cell resolves quicker with our injection patients.
Hemorrhagic occlusive retinal vasculitis (HORV). While not studied in the contralateral eye study, this is a severe ischemic hemorrhaging of retinal blood vessels research shows can happen after cataract surgery or intracameral injections. This rare but devastating pathology can cause bilateral no light perception vision. A retrospective case series report by the American Academy of Ophthalmology linked all cases of HORV to intraocular vancomycin.13
Clinician’s take: We no longer use intravitreal vancomycin, and the popularity of intracameral moxifloxacin continues to grow. We’ve managed hundreds of cataract patients with postop intravitreal injections, and have not had one case of HORV.
Retinal detachment. Anecdotally, clinicians were concerned of increased retinal tears and detachments with intracameral meds.
Clinician’s take: Unfortunately, retinal tears and detachments are an inherent risk with any cataract surgery, but in our practice we have not seen an uptick in these complications with the near total adoption of intravitreal Tri-Moxi.
Conclusion
Intravitreal and transzonular medications are improving patient compliance, reducing pharmacologic cost and increasing the post-op drop convenience—and these all positively affect patient satisfaction. In one study, patients show a statistically significant preference for injectable medications over postoperative drops.14 Optometrists are referring more patients for cataract surgery, and the option of offering fewer drops continues to gain steam with patients.14 According to the 2014 ASCRS Endophthalmitis Survey, 47% of American surgeons are using some type of intracameral injection after cataract surgery, and this number is up 20% from the data reported in 2007.15
In our practice, a common question from patients at the conclusion of a cataract evaluation is, “Can I do that injection so I don’t have to use so many drops?” The drop-a-day cataract surgical option with intravitreal postop medications has elevated both our patients’ experience and their satisfaction.
Dr. Ibach specializes in advanced anterior segment surgery care and pathology at Vance Thompson Vision in Sioux Falls, SD. He is a fellow of the American Academy of Optometry and a member of the American Optometric Association.
|
1. Lindstrom, R. Thoughts on Cataract Surgery: 2015. Rev. Ophthal. 2015;22(3):62-4. 2. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):288-94. 3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-42. 4. Braga-Mele R, Chang DF, Henderson BA, et al. Intracameral antibiotics: Safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-42. 5. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39(1):8-14. 6. Kessel B, Tendal B, Jorgensen K, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops. Amer Acad Ophthalmol. 2014;121(10):1915-24. 7. Angela J, Kasner O, Samek DA, et al. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cat Refract Surg. 2014;40(11):1857-61. 8. Vigamox and Prednisolone acetate 1%. Good Rx. www.goodrx.com. Accessed February 3, 2017. 9. American Academy of Ophthalmology. Cataract in the AdultEye PPP. October 2011. www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp--october-2011. Accessed April 28, 2017. 10. Packer M, Chang DF, Dewey SH. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714. 11. Barry P, Seal DV, Gettinby G, et al; for the ESCRS Endophthalmitis Study Group. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: case for a European multicenter study. J Cat Refract Surg. 2006;32:396-406. 12. Ibach M, Greenwood M, Schweitzer J, et al. Evaluation of a trabecular micro-bypass stent in combination with cataract surgery plus triamcinolone-moxifloxacin-vancomycin intravitreal injection. E-poster presented at American Society of Cataract and Refractive Surgery. May 2016. 13. Witkin A, Chang D, Jumper J, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis. Ophthalmology. 2017;124(5):583-95: 14. Fisher BL, Potvin R. Transzonular vitreous injection vs. a single drop compounded topical pharmaceutical regimen after cataract surgery. Clinical Ophthalmology. 2016;10:1297-1303. 15. Chang D, Braga-Mele R, Henderson B, et al. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: Results of the 2014 ASCRS Member Survey. J Cat Refract Surg. 2015;41(6):1301-5. |

