 Recent research has shed more light on the intensity of the diabetes epidemic in the US—indicating a 45% increase in the prevalence of the disease among American adults over the past two decades.1
Recent research has shed more light on the intensity of the diabetes epidemic in the US—indicating a 45% increase in the prevalence of the disease among American adults over the past two decades.1
Like many other specialties in health care, eye care has been ramping up its efforts in diabetes prevention and management. We also have to take a look beyond the eyes of our diabetic patients.
Optimal ocular health and wellness requires comprehensive evaluation at appropriate intervals and interprofessional cooperation among providers. Proper control and management of systemic disorders and metabolic abnormalities associated with diabetes influences the onset, progression and visual outcome of diabetic eye disease.
More Than Just Retinopathy
When we’re talking about diabetic eye disease, retinopathy and macular edema often dominate the conversation. And while these conditions certainly are the most frequent ocular manifestations of diabetes, they’re far from the only complications we have to monitor.
Individuals living with diabetes are 40% more likely to develop glaucoma and 60% more likely to develop cataracts than those without.2
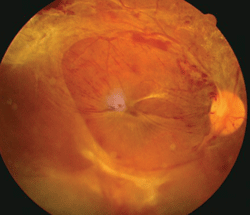
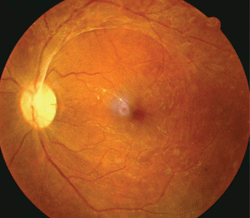
This patient exhibited proliferative diabetic retinopathy in both eyes. He had a hemoglobin A1c of 9%, body mass index of 45, obstructive sleep apnea, hypertension, vitamin D deficiency and a history of smoking.
More specifically, cataracts manifest earlier and progress faster in patients with diabetes, and the risk of glaucoma increases accordingly with duration of disease.3,4
This means double trouble for seniors age 65 and older—a demographic already prone to cataracts and glaucoma, but also identified as the age group with the greatest rise in diabetes prevalence over the last 20 years.1
Other ocular anomalies associated with diabetes include decreased corneal sensitivity, surface dryness, iris neovascularization, pupillary abnormalities secondary to autonomic neuropathy, fluctuating refractive error associated with sorbitol in the lens, a “snowflake” cataract in patients with type 1 diabetes, optic nerve abnormalities and other cranial neuropathies.5,6
An Interprofessional Approach
Scores of studies have supported the theory that optimal control over systemic and metabolic factors can effectively prevent the development and progression of diabetic retinopathy (DR) and diabetic macular edema (DME). However, many patients fail to achieve or maintain optimal levels of metabolic control.
As primary care optometrists, we can help by being aware of the associated factors that can modify (in either a good or bad way) retinopathy that may become sight threatening. If our patients appear to be at an elevated risk for any associated complications of diabetes, it’s our responsibility to raise a red flag and recommend that they seek the proper medical follow-up.
Factors That Modify Diabetic Eye Disease
Here is a breakdown of common associated systemic and metabolic comorbidities and factors that modify diabetic eye disease:7-12
• Hypertension. Systemic hypertension is an established risk factor, both for development and progression of DR. Diabetic patients with concomitant hypertension are up to three times more likely to develop DME. Patients’ blood pressure should remain below 125mm Hg/80mm Hg.
• Kidney disease. Changes in renal function may portend retinal changes. Normal blood urea nitrogen (BUN) to creatinine ratio should hover between 10:1 and 20:1. A person with diabetes and proteinuria (a.k.a., albuminuria) with or without high blood pressure may need a class of drugs called angiotensin-converting enzyme inhibitors, or a similar class called angiotensin receptor blockers. These drugs have been found to protect kidney function and possibly retinal tissue.
• Dyslipidemia. According to the American Diabetes Association, the target for LDL cholesterol level for men and women with diabetes is <100mg/dL. For HDL, the target is >40mg/dL for men and >50mg/dL for women.
• Anemia. Anemia often accompanies diabetic kidney disease. Hemoglobin levels should measure 12g/dL or higher.
More than a
decade ago, researchers in the UK found that subjects with type 2
diabetes had reduced macular pigment optical density (MPOD) compared
with non-diabetic controls.13
Additional
findings, which showed a relationship between reduced MPOD and
increased severity of diabetic maculopathy, may implicate oxidative
stress as a causative factor.13
A more recent study showed
that type 2 diabetes patients—with or without retinopathy—had reduced
MPOD compared scores to those of non-diabetic patients. In addition,
researchers observed a significant inverse correlation between MPOD and
HbA1c levels.14
A
series of studies illustrated how measuring crystalline lens
autofluorescence may help identify the presence of elevated advanced
glycosolated endproducts (AGEs)––a biomarker highly correlated to
glycemic control––prior to the presentation of early-stage
complications.
Subjects with poor glycemic control had significantly
higher levels of AGE in their lens compared to age-matched healthy
controls.13,15,16
This research has gained increased
relevance with the commercial availability of the first FDA-approved
noninvasive lens fluorescence biomicroscope (ClearPath DS-120, Freedom
Meditech), which measures autofluorescence via a six-second scan of the
crystalline lens.
• Obstructive sleep apnea. Obstructive sleep apnea may aggravate diabetic retinopathy and diabetic macular edema, secondary to nocturnal hypertension and hypoxemia. Obtain a pulmonology consult for sleep studies and treatment, if necessary.
Novel Ocular Biomarkers for Diabetes
Multiple
studies have indicated that serum carotenoids, including the macular
pigments lutein and zeaxanthin, are inversely associated with type 2
diabetes and impaired glucose metabolism.13,14
• Obesity. Obesity is a risk factor for both sleep apnea and type 2 diabetes. Discuss the importance of nutrition and physical activity with patients.
• Tobacco use. Smoking increases activated circulating leukocytes and platelet activation, and the nicotine in tobacco products causes severe retinal vasoconstriction. Provide information about local or statewide tobacco cessation programs to help patients kick the habit.
• Glycemic control. Help patients reach a goal of 6.5% hemoglobin A1c through proper nutrition, exercise, insulin use or oral medications.
• Vasculitis. Rule out gum disease, leg ulcers, gastritis and urinary tract infections.
• Neuropathy. Like retinopathy and nephropathy, neuropathy is a chronic microvascular complication. To prevent diabetic neuropathy, we must recognize the risk factors and the symptoms (numbness, tingling) and signs (reduced reflexes, poor nerve conduction), and understand the importance of tight glycemic control.
• Vitamin D deficiency. Boost patients’ vitamin D levels with protected exposure to sunlight and supplements, particularly in darkly pigmented individuals. Vitamin D insufficiency is more prevalent among blacks than individuals of other races. Further, most young, healthy blacks in North America do not achieve optimal 25-hydroxyvitamin D concentrations at any time of year. This is because increased skin pigmentation reduces vitamin D production in the skin.
• Insufficient sleep. Lack of sleep can lead to increased blood insulin, insulin resistance, cortisol release and inflammation. Encourage good sleep hygiene and recommend at least seven hours of uninterrupted sleep per night.
• Chronic stress. Chronically elevated stress levels can lead to a change in gene expression and cellular aging as well as an increase in cortisol, insulin and inflammation. Cortisol, in turn, increases cytokine production and oxidative stress. Suggest stress reduction and a balanced lifestyle.
It is crucial that health care providers interact with one another in managing our patients to ensure that they achieve the best quality of life and health. As primary health care providers, we must go beyond the retina in caring for patients with diabetes.
1. YJ, Imperatore G, Geiss LS, et al. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988-2010. Diabetes Care. 2013 May 1.
2. American Diabetes Association. Living With Diabetes: Eye Complications. Available at:
www.diabetes.org/type-1-diabetes/eye-complications (accessed July 27, 2012).
3. Chopra V, Varma R, Francis BA, et al. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008 Feb;115(2):227-32.e1.
4. Leske MC, Wu SY, Hennis A, et al. Diabetes, hypertension, and central obesity as cataract risk factors in a black population. The Barbados Eye Study. Ophthalmology. 1999 Jan;106(1):35-41.
5. Negi A, Vernon SA. An overview of the eye in diabetes. J R Soc Med. 2003 Jun;96(6):266-72.
6. Rogell GD. Corneal hypesthesia and retinopathy in diabetes mellitus. Ophthalmology. 1980 Mar;87(3):229-33.
7. Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema pathophysiology, screening, and novel therapies. Diabetes Care. 2003 Sep:26(9):2653-64.
8. Pelino CJ, Pizzimenti JJ. Contemporary care protocols for DR and DME. Rev Optom. 2012 Aug;149(8):90-9.
9. Wagner H, Pizzimenti, JJ, Daniel K, et al. Eye on diabetes: a multidisciplinary patient education intervention. Diabetes Educ. 2008 Jan-Feb;34(1):84-9.
10. Chiodini I, Adda G, Scillitani A, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007 Jan;30(1):83-8.
11. Salpea KD, Humphries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010 Mar;209(1):35-8.
12. Touma C, Pannain S. Does lack of sleep cause diabetes? Cleve Clin J Med. 2011 Aug;78(8):549-58.
13. Davies NP, Morland AB. Color matching in diabetes: optical density of the crystalline lens and macular pigments. Invest Ophthalmol Vis Sci. 2002 Jan;43(1):281-9.
14. Lima VC, Rosen RB, Maia M, et al. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: a comparative study. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5840-5.
15. Yu NT, Krantz BS, Eppstein JA, et al. Development of a noninvasive diabetes screening device using the ratio of fluorescence to Rayleigh scattered light. J Biomed Opt. 1996 Jul;1(3):280-8.
16. Sparrow JM, Bron AJ, Brown NA, Neil HA. Autofluorescence of the crystalline lens in early and late onset diabetes. B J Ophthalmol. 1992 Jan;76(1):25-31.

