Annual Cornea ReportFollow the links below to read other articles from our annual Cornea Report: An OD’s Guide to Corneal Transplant Options |
Conquering corneal infiltrates is something clinicians have attempted to do for decades. Despite continued research and elevated clinical acumen, if you were to put 20 clinicians in front of 20 slit lamps and ask them to properly distinguish between sterile and infectious corneal infiltrates (whether bacterial, viral, fungal or protozoan), you would hear many differing opinions.
Perhaps that is because the question itself is inherently flawed—an infiltrative process accompanies every infection. Likewise, a loss of stromal substance in corneal ulceration is often (but not always) accompanied by an infectious process. Some infections (e.g., fungal or protozoan keratitis) have an intact epithelium even though an infectious process is at play. Therefore, both infiltrates and ulcers can be either sterile or infectious. While it is true that diffuse infiltrates with little to no epithelial involvement are commonly sterile, clinicians need to avoid over-reliance on the rule of thumb that ulceration signifies infection and infiltrates do not.
Instead, optometrists must rely on patient history, symptoms and clinical presentation when determining the type of corneal infiltrate. Once we know what we are dealing with, only then can we choose the proper treatment and management regimen. Here is a look at the processes that lead to infiltrates and how to distinguish the various types you will encounter in your practice.
 |
| Fig. 1. These subepithelial infiltrates are a hallmark sign of EKC. Click image to enlarge. |
How it All Begins
Corneal infiltrates represent an immune response to corneal insult, whether from a microbial antigen, contact lens wear or even corneal surgery. A firm grasp of corneal mechanics is a first important step toward understanding how an infiltrate occurs.
The cornea, devoid of blood or lymph vessels, relies on cellular and molecular properties within it and the surrounding tissue.1 Corneal epithelial cells are an important asset in activating the immune response. As the first line of defense, corneal epithelial cells identify an invading pathogen or other corneal insult and release cytokines and chemokines to begin the immune defense. In addition to epithelial cells, a variety of other players are also key in the corneal defense mechanism, including keratocytes, interferons, neutrophils, natural killer cells and macrophages.2 The active immune response involves immune cells arriving to repair the corneal damage, eventually leading to the aggregation of white blood cells in the cornea—known as an infiltrate.3
Although every infection has an infiltrative process, not every infiltrate is infectious. One of the important distinguishing factors between sterile and infectious infiltrates is the status of the epithelium. With an intact epithelium, more often the infiltrates are sterile; with an infectious etiology, a defect is usually present (Table 1).4 The most important information needed to diagnose infiltrative events arises through patient history and presentation (Table 2).
Table 1. Sterile Infiltrates vs. Infectious Infiltrates4 | |
| Sterile | Infectious (MK) |
|
|
Table 2. Clinical Characterization of Corneal Infiltrative Events with Soft Contact Lens Wear11 | ||
| Classification | Categories | Signs and Symptoms |
| Serious and symptomatic | Microbial Keratitis |
|
| Clinically significant and symptomatic | Contact lens-induced acute red eye (CLARE) |
|
| Contact lens peripheral ulcer (CLPU) |
| |
| Infiltrative Keratitis |
| |
| Clinically non-significant and asymptomatic | Asymptomatic infiltrative keratitis |
|
| Asymptomatic infiltrates |
| |
 |
| Fig. 2. Above, this infiltrate, known as a Wessely ring, was caused by a bacterial source. Below, the migrating stromal white blood cells, seen as an area of granularity on the edge of retro beam, are a response to the infiltrate. Photos: Aaron Bronner, OD |
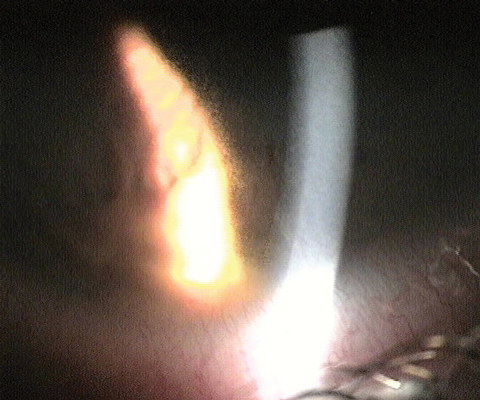 |
Infections: The Usual Suspects
Although patient history and presentation will provide a better understanding of the initial antigen causing the infiltrate, it is best to proceed with caution in diagnosis and treatment.
Because it is difficult to clearly differentiate between a benign, self-limiting corneal insult and an infectious event, clinicians should first treat all infiltrative events as infectious in nature.5 Any number of different infectious etiologies may be at play, and knowing what you are looking at is crucial. Let’s review the most common infectious causes of corneal infiltrates.
• Viral subepithelial infiltrates. Adenoviruses—including epidemic keratoconjunctivitis (EKC), herpes simplex virus (HSV) and herpes zoster (HZO)—can have significant corneal involvement. Some patient history questions that can help narrow the diagnosis include whether they feel fatigued and if they had an upper respiratory infection recently.
• EKC. This often presents bilaterally, and patients sometimes have a history of respiratory tract infections. Around one week to 10 days after inoculation, patients may develop follicular conjunctivitis, petechial hemorrhages, prominent preauricular adenopathy, occasionally pseudo- or true membranes and, often, associated punctate keratitis. These patients commonly complain of tearing, light sensitivity, pain and foreign body sensation. Seven to 14 days after their initial eye symptoms, patients may develop multifocal subepithelial (anterior stromal) corneal infiltrates (Figure 1). These become quite apparent on slit lamp examination and can range from a few to many.6
The punctate erosions (keratitis) arise due to adenovirus replication within the corneal epithelium, whereas the infiltrates are due to an immunopathologic response to a viral infection of keratocytes in the superficial corneal stroma. Patients who complain of photophobia and decreased vision as a result of the SEIs often have symptoms that persist for months after the initial presentation.6
In the case of EKC, laboratory testing is rarely indicated. However, a viral culture may differentiate between EKC and a herpes simplex virus (HSV) infection.
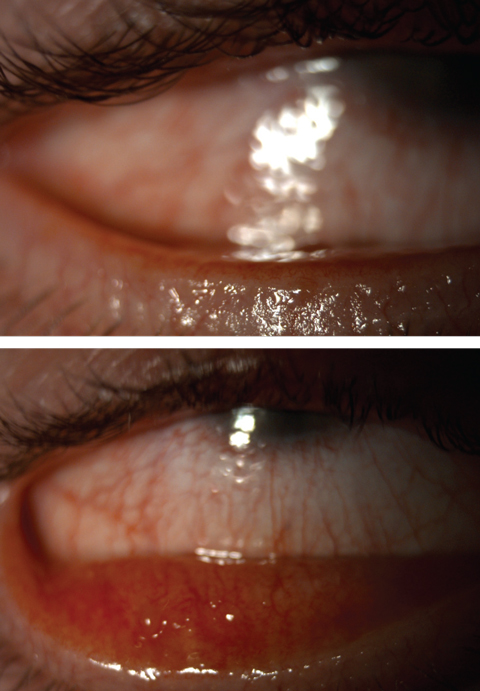 |
| Fig. 3. Severe meibomian gland dysfunction with telangiectatic vessels. Click images to enlarge. |
• HSV and HZO keratitis. These are the most commonly known viral keratitis infections other than EKC. Patient history questions pertaining to previous infections such as chicken pox, inordinate stress or recent sun exposure are helpful to identify this etiology.
HSV comes in various forms that can affect different layers of the cornea, ranging from epithelial, neurotrophic, necrotizing stromal and endotheliitis. Epithelial keratitis can present with blepharoconjunctivitis—macropunctate epithelial lesions that progress to dendritic ulceration with terminal end bulbs.7 Anterior stromal haze can develop “ghost dendrites” below the epithelial lesions. HSV can also have a non-necrotizing and necrotizing stromal keratitis. This appears differently than a small infiltrate, as there is a central disc of stromal or epithelial edema, keratic precipitates and endothelial folds. Associated signs also include anterior uveitis, decreased corneal sensation and elevated intraocular pressure.8
HZO may present with epithelial keratitis comprised of small, nonulcerated pseudodendrites without terminal bulbs.7
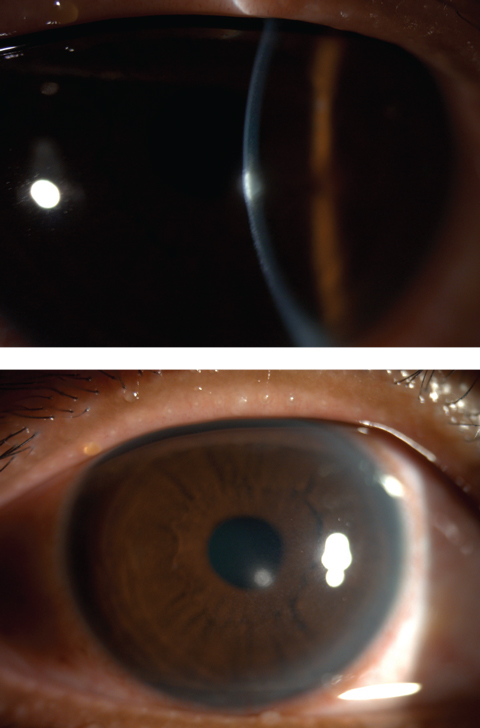 |
| Figs. 4 and 5. Central corneal infiltrates from contact lens wear. Click image to enlarge. |
• Bacterial keratitis. This can involve a suppurative corneal infiltrate with an overlying epithelial defect after a bacterial corneal insult (Figure 2). The ocular flora is home to both Staphylococcus and Streptococcus, the most common agents found in opportunistic infections due to ocular surface trauma (e.g., corneal abrasion, surgery, severe ocular surface disease). In contact lens wearers, however, bacterial keratitis is most often due to Pseudomonas.9,10 Although contact lens infiltrates are usually thought to be “sterile,” on culture they can be either sterile or infectious; thus, infiltrates in a patient who wears contact lenses should be treated as infectious until proven otherwise.9
Bacteria often reside in contact lens cases, patient’s hands, eyelids and in tap water; basically any entity that comes in contact with the lenses can cause a bacterial infection.11 The antigens are trapped between the contact lens and cornea, remaining on the cornea longer due to slow epithelial cell renewal.12 The benefit with rigid gas permeable (GP) lenses is the increase in tear exchange vs. soft lenses, which have 10 to 20 times higher incidence of infiltrative events.10,12 GPs are also considerably more deposit resistant, reducing a key predisposing factor for infection.
Asking contact lens patients about wear time and lens hygiene are important points in the history, as these two are commonly associated with bacterial infections from contact lens wear.12 Extended contact lens wear carries a 43% risk of bacterial keratitis.12 Hypoxia because of overnight wear, extended wear or hypersensitivity to lens material triggers the inflammatory response, leading to the formation of an infiltrate. The infiltrate is irregular with surrounding corneal edema, and is usually described as greater than 1mm, with the possibility of adjacent satellite lesions.11,12 A deep corneal defect extending from the epithelial layer into the stroma with necrotic tissue is also present. The infiltrate is usually located in the central or paracentral cornea.
With a Pseudomonas infection in particular, a large central defect can be present.9 Symptoms often include severe pain, severe redness, photophobia, hypopyon, marked anterior chamber reaction, mucopurulent discharge and decreased visual acuity.
• Fungal keratitis. This is less common than bacterial keratitis. It represents around 5% to 10% of corneal infections in the United States. The leading cause of fungal keratitis is trauma to the cornea with plant or vegetable material; however, contact lenses are another risk factor. Clinicians should ask patients about their recent activities outdoors in addition to their contact lens habits.13
These infections usually present with fewer symptoms than bacterial keratitis, but a deep stromal gray-white dry infiltrate with a feathery margin may be present. If the infiltrate is extremely deep, it is possible to have multifocal or satellite infiltrates, endothelial hypopyon or both. It is very important to distinguish this type of infiltrate from other forms, as topical corticosteroids are a significant risk factor. They can activate and increase the virulence of the fungal organism, which results in a decrease in resistance to the infection.13
• Protozoan infections. The parasitic infection we hear most about in eye care is Acanthamoeba. Diagnostic delay is common because of the nonspecific presentation. Presenting symptoms are often severe ocular pain and photophobia. In the early stages, a localized infection may appear in a mildly symptomatic patient with diffuse punctate epitheliopathy or dendritic epithelial lesion. A gray-white superficial infiltrate may occur in the central cornea. This infection can progress into a partial or complete ring infiltrate. It is important to question these patients if they have worn their contact lenses in a pool, hot tub or fresh water source. A diagnosis can be made by using a stained smear or by culturing organisms from the corneal scraping. Most cases are diagnosed by clinical presentation or confocal microscopy.13
 |
| Fig. 6. While this grid is a simplification for categorization of an infiltrative event, it functions as a good starting point in identifying the infiltrative cause. |
Non-infectious Infiltrates
If you have ruled out an infectious etiology, be on the lookout for several sterile infiltrative events: You should begin by examining the company the infiltrate keeps—lid margin disease and blepharitis are often found in conjunction with non-infectious infiltrates.
• Marginal corneal infiltrates. These are caused by non-infectious conditions. Although the complete pathological process of these infiltrates is still not fully understood, researchers do know that Staphylococcus grows on the eyelids and spills bacterial byproducts onto the corneal surface, beginning a hypersensitivity reaction thought to lead to infiltrates.14
The introduction of an antigen onto the cornea surface will cause a release of inflammatory mediators to the peripheral cornea, leading to vasodilation. The corneal limbus is very important in immune-mediated corneal disorders because it has antigen-presenting cells (APCs), such as Langerhan cells, that express major histocompatibility complex class II antigens, which are capable of efficient mobilization and induction of B- and T-cell responses. This is why immune-mediated corneal changes occur at peripheral locations adjacent to the limbus.15
These sterile corneal infiltrates are often small, gray-white circumlimbal lesions separated from the limbus by a 1mm clear space. The location of these infiltrates is due to the APCs that are capable of mobilization of the T-cell response. In addition, the posterior limbus is vascularized, circulating immune cells and complexes near the terminal capillary loops, causing a variety of immune responses in the corneal periphery.15 If the infiltrates are due to chronic Staphylococcal lid disease, superficial blood vessels may occur across the clear interval between the limbus and the infiltrates (Figure 3).15 They more commonly appear individually, but can appear in groups or bilaterally.16 Infiltrates may be non-staining or have early overlying staining, and are usually present where the eyelid margin intersects the corneal surface (i.e., at the 2 to 10 o’clock and 4 to 8 o’clock areas).15
Slit lamp exam may also reveal mild quadrant-specific conjunctival hyperemia, little or no chemosis, trace or mild ocular irritation and normal vision. These infiltrates are self-limiting and usually disappear within one to two weeks.15
Clinicians should always be on the lookout for masqueraders as well. Krachmer’s spots, for example, are a type of sterile infiltrate that can be mistaken for either marginal or viral subepithelial infiltrates.17 These are a sign of subepithelial corneal graft rejection after penetrating keratoplasty, and patients may present asymptomatically. Research has yet to determine if the lymphocytic cells arise from donor keratocytes or the donor epithelial cells. These infiltrates can be accompanied by an anterior chamber reaction. If infiltrates are seen in a post-corneal transplant patient, it is important to rule out subepithelial rejection.10
• Contact lens-induced infiltrative events. Infiltrative events can also be specifically associated with contact lens use (Figures 4 and 5). A lens wearer’s habits will help clinicians better understand the possible cause of an infiltrative event. Proper follow-up questions include: Which type of lens? How often? For how many hours? Are you practicing appropriate lens hygiene? Do you sleep in them? Do you change them regularly? What solution do you use? Do you swim in them? With the appropriate questions during patient history, a diagnosis, such as Acanthamoeba, should begin to form (Figure 6).
• Contact lens-induced peripheral ulcer (CLPU) is a sudden corneal inflammatory response after contact lens wear that presents with moderate-severe limbal and bulbar redness (Figures 7 and 8).5 A small circular subepithelial infiltrate is often present in the periphery or mid-periphery as well (0.1mm to 1.2mm in diameter).5 There is an associated epithelial defect with surrounding infiltrates. Presenting symptoms can include moderate-to-severe pain, foreign body sensation and irritation, or patients may present asymptomatically.11 CLPU is self-limiting and will resolve after discontinuing contact lens wear. There can be recurrences with contact lens wear; however, proper lens hygiene is stressed to prevent further inflammation.
• Contact lens-induced acute red eye (CLARE) also an inflammatory reaction of the cornea, often occurs after sleeping with lenses overnight. Patients will present with pain upon awakening, photophobia and tearing.11 Multiple focal infiltrates of 1mm in diameter or less can be present in the mid-periphery or periphery of the cornea.13 There is minimal staining and no epithelial defect present on examination.18 Resolution typically occurs quickly after lens removal.
• Infiltrative keratitis. This condition is an inflammatory reaction with infiltrates occurring in the anterior stroma. An epithelial defect can be present, but is not a certainty. Infiltrates are located in the corneal mid-periphery or periphery and are smaller in size, usually less than 1mm in diameter.18 Patients may present with symptoms of irritation and redness.11 Infiltrative keratitis can also occur with asymptomatic patients, where smaller infiltrates, 0.4mm in diameter, are present in the corneal periphery.11 Punctate staining may be present with mild redness, which differentiates this from asymptomatic infiltrates. As opposed to CLPU and CLARE, this occurs during the day and the focal infiltrates are irregular.
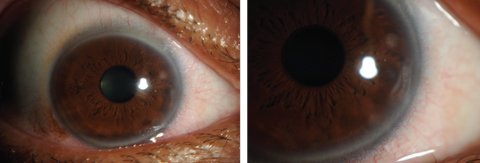 |
| Figs. 7 and 8. Contact lens-induced peripheral corneal infiltrates. Click images to enlarge. |
Differentiation is Key
Classifying infiltrates as sterile or infectious is a challenging task, and differentiating their underlying etiologies can be complicated due to the multiple potential causes and their often-overlapping signs and symptoms. Patient presentation and a thorough case history will provide crucial information needed to narrowing down the diagnosis. Once the most likely etiology of the infiltrate has been determined, appropriate treatment can be undertaken. With infectious etiologies in particular, the case should be approached with the most up-to-date protocols for treatment or the appropriate consultation or referral.
Dr. Sherman is an instructor in Optometric Science (in Ophthalmology) at Columbia University Medical Center. She received her undergraduate degree from the University of Michigan and graduated from SUNY College of Optometry. She completed her optometric residency in ocular disease and primary care at Bronx Lebanon Hospital Center. She specializes in complex and medically necessary contact lens fittings and ocular disease.
Dr. Shuja is an optometrist at New York-Presbyterian Hospital. She received her undergraduate degree from SUNY at Stony Brook and her optometry degree from Pennsylvania College of Optometry at Salus University. She completed her residency at the Northport Veteran Affairs Medical Center.
1. Kumar A, Yu F-SX. Toll-like receptors and corneal innate immunity. Current Molecular Medicine. 2006;6(3):327-37. |

